The Use of Silicone Adhesives in Space Applications
Every single design consideration—including the adhesives selected—is important in the costly endeavor to send an object into space.

The harsh conditions of space place a set of constraints on the technologies that take us there. Of primary concern when it comes to space system design is the adhesive chosen to hold everything together. What does it take to perform in the vacuum of space, and why will silicone adhesives continue to be the adhesive of choice in many aerospace applications?
The conditions of space impose a unique set of constraints on the technologies that take us there. Indeed, the rigors of space exploration have often been the driving force behind many new technological innovations. Far from the insulating layers of the atmosphere, temperatures can reach as low as -200˚F in the shade and as high as 200˚F when facing the sun. Combine this with a vacuum environment and add to it extremely sensitive electronic and optical devices, and the challenges are daunting. Yet the successes and failures of the past have only increased man's desire to expand his vision of the stars.
Every single design consideration is important in the costly endeavor to send an object into space. One of these considerations, which at first may not appear to be large but can lead to catastrophic results if poorly considered, is the choice of adhesive to be used in various components of space systems. These adhesives are used for bonding, insulating, damping, conducting, and a host of other reasons. They are often used near or directly adjacent to electronic optical devices where contamination would be of major concern. In general, any material used in space must possess the following characteristics:
- Good resistance against radiation degradation
- Outstanding atomic oxygen resistance
- Excellent micro-cracking resistance against thermal cycling
- Low outgasing characteristics
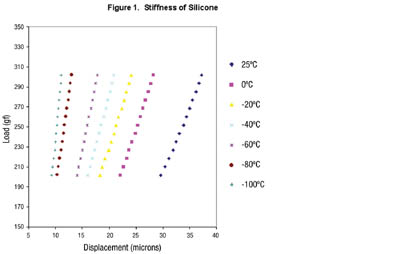
Figure 1 shows the displacement of a low outgassing silicone at various small loads for temperatures approaching -150˚F. Stiffness is calculated from the slope of the line. Silicone maintains some degree of flexibility at -150˚F, a temperature at which many other polymers would become brittle or crack. [Data obtained from Johns Hopkins APL using ARLON Thermabond].
Resin systems that meet some of these requirements include polyimides, cyanate esters, silicone, epoxy, polyurethane and acrylic resins. Among the adhesive polymers available for an aerospace materials engineer, silicone stands out as possessing certain obvious advantages. Phenyl-based silicones are able to withstand the temperature extremes that are experienced in space and are able to maintain a good degree of flexibility at very low temperatures where other materials would stiffen and crack (see Figure 1). Special grades of silicones have brittle points below -150˚F. The elastomeric properties dampen the effects of launch vibration. Silicone is also used as an adhesive to bond materials that have dissimilar coefficients of thermal expansion (CTE). Differences in CTE become a problem when two bonded surfaces experience the dramatic temperature changes encountered during space flight. These temperature changes cause one surface to contract or expand more than the other. In the case of circuit boards that are bonded to metal heat sinks, stresses on the circuit board could cause solder joint failure and lead to device malfunction.
Like all other polymers, silicone is susceptible to radiation that exists in space due to the absence of any atmospheric gases that would serve as an obstacle in the path of electromagnetic waves and subatomic particles. While radiation can cause changes in the properties of silicone rubber similar to those caused by heat aging, certain specialty silicones possess excellent resistance against radiation (see Table 1). In addition to radiation, atomic oxygen is another factor with which aerospace materials engineers must deal. Atomic oxygen reacts with polymers causing erosion, which is a threat to spacecraft durability. Nearly 90% of the atmosphere from 100 to 350 miles from the Earth's surface is comprised of atomic oxygen. Silicone adhesives would generally not be exposed to this form of oxygen, as they would be encapsulated between two surfaces; however, silicone has been used as a coating in some space applications to protect various polymer substrates.
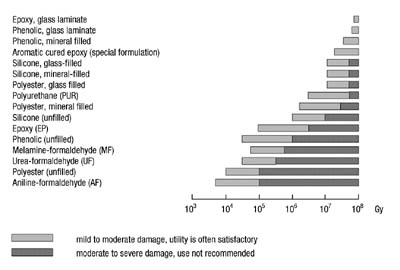
The most significant drawback to silicone's use in space applications has been outgassing. Untreated silicones generally contain volatile species that can outgas and contaminate sensitive optics. NASA contamination guidelines use a test method (ASTM E595) which gives the maximum allowable amounts for a material to be considered as low outgassing. There are two components to this specification, the total mass loss (TML) and the collected volatile condensable materials (CVCM). Total mass loss is simply the amount of material given off during a 24 hour time period at 125˚C and less than 5 x 10-5 torr. CVCM is the amount of volatiles that will condense on a collector controlled to 25˚C. For aerospace applications, TML must be less than 1%, while CVCM must be less than 0.1%. Standard silicones can yield more than 5% TML and 1% CVCM.
Outgassing of silicone depends on what grade of silicone is used. In general, the volatiles are low molecular weight polydimethylsiloxanes, which are the remnants and products of the polymerization reaction. Siloxanes with chain lengths of 4 to 10 siloxy groups constitute what are commonly referred to as "total silicone volatiles."1 The number of volatiles that outgas depends not only on the type of silicone used but also on the type of non-polymer filler, additives and curing agents. Processing also plays a role in determining the final properties of any given formulation. Fortunately, the use of special formulation and processing techniques results in a silicone adhesive that meets the requirements of NASA outgassing specifications and retains all of the adhesive benefits that silicone provides.2 Specialty silicones employing optimized cure cycles have been able to achieve as low as 0.06% TML and 0.02% CVCM.
Silicone adhesives are currently being used in a variety of space applications. Electronics for a satellite constructed by the Johns Hopkins University Applied Physics Laboratory for the NASA Goddard Space Flight Center required a silicone adhesive suitable for extremely cold temperatures in order to bond heat sinks to PCBs. This example serves to highlight another unique condition of space - for internal devices, conduction is the only method for heat transfer since there is obviously no convection of air, and radiation would only occur at the surface of the spacecraft. Another example is the use of a silicone adhesive to bond a radiation conduction bar to the mount plate of a charged coupling device (CCD) of a telescope. The proximity of the silicone to the CCD required the development of a very low outgassing formulation since any volatiles could contaminate the device and diminish the telescope's capability. These and other examples show that silicone will increasingly be an adhesive of choice in many aerospace applications.
For more information on silicone adhesives, contact Kareem M. Monib, ARLON, Silicone Technologies Div., phone 302-595-1231; e-mail kmonib@arlon-std.com; or visit www.arlon-std.com.
References
- Toube , Mel. Factors Affecting Silicone Volatile Levels in Fabricated Silicone Elastomers. Rubber World, June 2002, Vol. 226, No. 3.
- See Thermabond in NASA outgassing data at www.outgassing.nasa.gov.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







