
Release liners based on silicone (PDMS) chemistry are used extensively in the tape market. Liners serve several useful functions: they are used as a carrier sheet onto which the adhesive is cast; they protect the adhesive during storage and transit; they provide a functional support during die-cutting and printing; they carry useful product instructions; and they ultimately release from the adhesive leaving it undamaged. Most importantly, the release material must not compromise the subsequent performance properties of the tape.
Several technical parameters must be met in order to achieve these uses. A basic requirement for silicone-coated release liners is that the release liner surface must be coated uniformly with a continuous layer of silicone and that the silicone layer is well cured. This avoids any interaction that may occur between unreacted silicone species and the adhesive, and it minimizes the possibility of transfer of silicone to the adhesive surface. The level of release also must be adjusted properly, as defined by the customer's end-use requirements, to give consistent release values over time. Finally, the silicone coating must be well anchored to the liner substrate. This is particularly important if the liner is not used immediately, but rather is stored for a period of time prior to its use. Prolonged storage of release liners can lead to a degradation of the anchorage of the silicone. This is particularly true with silicones on film substrates, but can occur on paper as well.
This article will discuss aspects of silicone anchorage on paper substrates, and will examine a case study based on a splicing tape application in which the adhesive can affect the anchorage of the silicone. A mechanism to account for the loss of silicone anchorage will be proposed.
A Review of Silicone Chemistry
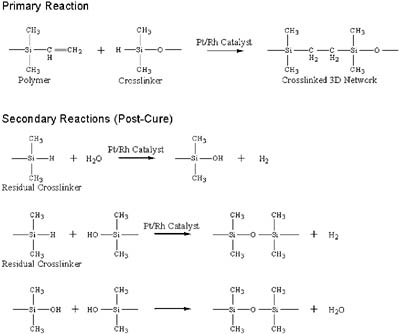
Silicone chemistries used for release coatings can be classified as either thermally cured or radiation-cured systems. Thermally cured systems can be classified further according to their curing mechanism as either condensation or addition (hydrosilylation) cure systems. Figure 1 shows the curing reactions of the addition cure chemistry. The primary crosslinking reaction occurs between a vinyl functional silicone polymer and a silicon-hydride functional crosslinker in the presence of a heavy metal catalyst such as Platinum (Pt) or Rhodium (Rh) (see reaction #1). Secondary reactions also occur1-2, especially when silicone systems are formulated with an excess of crosslinker (SiH). The first of these is the catalyzed hydrolysis of the SiH groups to form silanol (#2). The newly formed silanol group (SiOH) then catalytically reacts with any remaining SiH groups to form a type of crosslinking via the ~Si-O-Si~ bonds (#3). Finally, a condensation reaction of two silanol groups can form a ~Si-O-Si~ linkage (#4). Reaction 1 is referred to as the "crosslinking" or "cure" reaction while reactions 2-4 are jointly referred to as "post-cure" reactions, which occur as the material ages after coating. The platinum catalyzed reactions in Equations 2-3 are slower than the crosslinking reaction (1). Also, the silanol condensation reaction (4) is even slower than the SiH reactions (2 and 3). Comprehensive reviews of hydrosilylation chemistry can be found in the references.1,3-4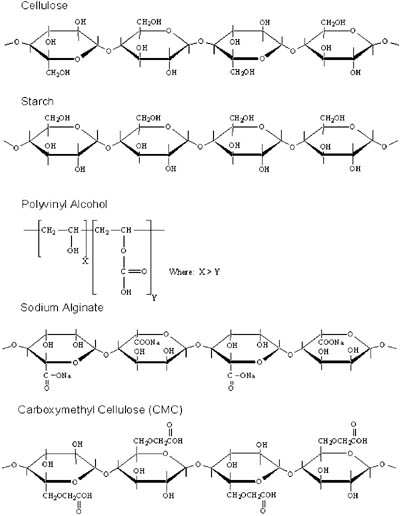
Silicone Adhesion
Silicones, by their chemical nature, are low surface energy materials. Their low surface energy is due to low polarity and a highly flexible backbone structure. Silicone release coatings typically have surface energies on the order of about 21-25 dynes/cm.5For these reasons, obtaining good anchorage to flexible substrates can present a challenge to release liner producers.Silicones anchor to substrates by way of two mechanisms: Mechanical interlocking with a substrate and chemical reactions with a substrate. Both mechanisms contribute to the anchorage of silicones. Mechanical interlocking occurs when silicones are applied to semi-porous substrates such as paper. Silicones flow very readily and can penetrate easily into porous papers, creating a mechanical "bond." However, most paper substrates used for siliconizing, such as glassine and supercalendered kraft (SCK), are designed specifically to keep the silicone coating on the paper surface and minimize surface penetration. This improves surface coverage and decreases silicone usage. Even with minimal surface penetration, mechanical interlocking still occurs between the silicone and the substrate. Mechanical interlocking, however, cannot account exclusively for long-term stable anchorage of silicones. It is the chemical interaction between the silicone coating and the substrate that accounts for the majority of silicone anchorage.
Paper substrates used for siliconizing are chiefly composed of cellulose fibers, the majority of which are derived from wood-based pulps. The two grades of paper mentioned above (i.e., SCK, glassine) also contain other ingredients such as sizing agents, which improve resistance to water or ink penetration. These are added directly to the pulp and are referred to as "internal sizes." In addition to the internal size, paper grades also contain surface coatings that are applied later in the paper-making process. These surface coatings are referred to as "surface sizes" and are based mainly on starch, polyvinyl alcohol, sodium alginate, carboxymethyl cellulose (CMC) or combinations thereof. Surface sizes are chiefly used as barrier coatings to decrease the surface penetration of the silicone into the paper and improve surface smoothness. Figure 2 shows the chemical structure of cellulose and the surface-sizing agents mentioned above.
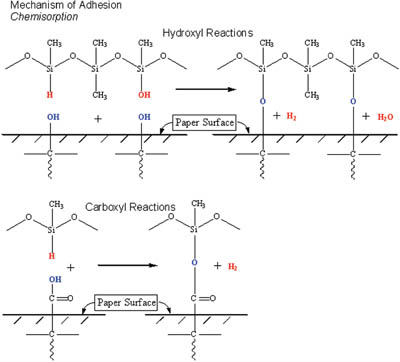
Most addition cure silicone systems (as illustrated in Figure 1) are formulated with an excess of silicone hydride crosslinker. Typical SiH/Si-vinyl ratios for these systems are on the order of 1.2:1 to 2:1, thus leaving unreacted SiH species after the primary crosslinking reaction (cure reaction) has occurred. The remaining unreacted SiH or SiOH groups from the hydrolyzed crosslinker (see reaction 2) can react with the various groups found on the paper surface. A silyl ether linkage is formed between surface OH groups and residual crosslinker. The COOH functionality of the substrate can form an silyl ester bond and impart silicone anchorage. Although these reactions are slower than the primary curing reaction, they do occur quickly. At speeds used for liner production, the silicone anchorage is firmly established by the time the liner is quality tested. No improvement in silicone anchorage is typically seen over time.
Next, a case study will be presented that will show that the bond formation between a silicone and a paper substrate can be disrupted due to the effect of an adhesive.
Case Study: Repulpable Splicing Tape
When adhesive tapes are manufactured, the tape manufacturer will typically cast adhesive directly onto the siliconized liner. The liner acts as a functional support for the adhesive during the curing process. After curing, a face stock is then laminated to the adhesive, creating a pressure-sensitive laminate. In some cases, however, the release liner is supplied with silicone coating on both sides, and, after adhesive coating and curing, the adhesive is wound up into a roll. Release liners supplied for this application generally have a tight release silicone on the side that the adhesive will be cast onto, and an easy release on the opposite side, thus making it easier to unwind. Adhesives in this application can be either unsupported or supported with a thin reinforcing layer sandwiched between two adhesive layers. Supported adhesives find applications as carton sealing, seam tapes and splicing tapes.A common application where both supported and unsupported adhesives are used is as a paper-splicing tape. Paper splicing tape is used in the paper industry for making flying splices during paper reel changes. Requirements for this product include the following.
- The tape must work for splicing different weights of paper during high-speed operation.
- The tape must bond instantly to paper stock over a wide range of temperature and humidity conditions.
- The tape is typically colored to allow for easy splice identification during subsequent processing.
- The tape is repulpable per TAPPI UM-213.
Recently, a problem was discovered in a commercial grade of paper used in a repulpable paper-splicing tape application. The liner consisted of a 78# bleached SCK paper silicone coated on both sides. Both sides use an easy release addition-cured silicone system. After an initial screening of release liners, the product was scaled up to commercial quantities. After adhesive coating, a loss of silicone adhesion was observed by the customer. The failure was characterized by the fact that both silicone release layers rubbed off easily from the liner surface after storing the adhesive coated rolls for several weeks. Initially, the loss of silicone anchorage was observed on the side onto which the adhesive was cast, but later both sides of the liner showed silicone rub-off. Silicone anchorage is checked using a digital rub test.
A search for the mechanism of failure was undertaken. A combination of surface analysis techniques was employed to investigate the complaint, including X-ray photoelectron spectroscopy (XPS) and Time-of Flight Secondary Ion Mass Spectroscopy (ToF-SIMS). Heat aging the silicone/adhesive laminate for one week at 70°C duplicated the rub-off issue. The customer provided adhesive coated samples for analysis. The samples were prepared and stored under two different conditions: 6 weeks at 20°C room temperature (RT) storage and 1 week at 70°C elevated temperature (ET) storage.

1. Silicone/Adhesive. The silicone surface in contact with the adhesive.
2. Adhesive/Silicone. The adhesive surface in contact with the silicone.
3. Paper/Silicone interface. The paper surface after silicone removal.
To examine the paper/silicone interface, the samples were carefully prepared by lightly scraping away the silicone layer to expose the paper/silicone interface. The chemical composition of each of these interfaces is summarized in the table.
For all the samples tested, the materials aged at elevated temperature (ET) exhibited silicone rub-off. No rub-off was seen with any of the room temperature (RT) aged samples. The silicone surface was examined after adhesive removal and was found to be PDMS for both cases with no presence of any contaminants.
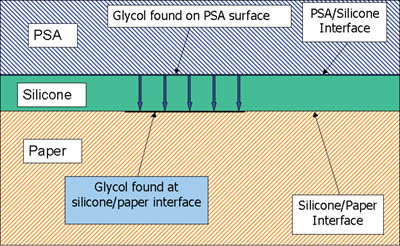
The adhesive surface was then examined. XPS analysis detected silicone on the adhesive surface: RT = 1.0% Si (atomic %) and ET = 4.6% Si (atomic %). It is not uncommon for silicones to show some transfer to an adhesive surface after laminate aging. Typically, even a well-cured silicone system will show some transfer of silicone to an adhesive, the percentage of transfer being a function of the aging conditions, chemical composition of the adhesive and the silicone system. More importantly, however, was the detection of polypropylene glycol (PPG) on the adhesive, which dominated the surface composition.
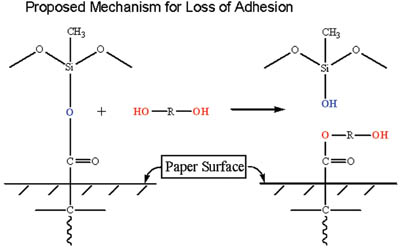
A discussion of the results with the customer indicated that the adhesive could not be reformulated, therefore the silicone/liner construction needed to be redesigned. Several design parameters were needed:
- No silicone rub-off after long term aging.
- An easy release level must be maintained on both sides of the release liner.
- The liner substrate must remain paper.
Two approaches were tried, the first involving a reformulation of the silicone system to prevent migration of the glycol to the paper/silicone interface. Several attempts were made, and it was found that in all cases the glycol component of the adhesive migrated through the silicone layer and caused a debonding of release coating.
The second approach involved maximizing the silicone/substrate reactions through reformulation of the silicone system and through manipulation of the manufacturing conditions of the release liner. By careful selection of the polymer, crosslinker and stoichiometry, this approach led to a solution to the problem, even though the presence of glycol was still measured at the interface.
Conclusion
Release liners are an integral part in the manufacture of pressure-sensitive tapes. Their use in the adhesive tape industry presents unique challenges for the release liner manufacturer.Long-term stability of release liners is fundamental to their successful use in the tape market. Properties such as release stability and silicone adhesion are critical parameters in the design and manufacture of release liners. Key to this is understanding the chemistry of the release system and the interactions that could take place with liner substrates.
Adhesives that contain migratory components can, in some cases, cause a loss of adhesion of the silicone release coating. By combining the right selection of silicone polymers and crosslinkers, and by selecting the optimum processing conditions, overcoming the loss in adhesion can be achieved.
Acknowledgements
The author wishes to thank Hans Schneiders for his contributions to this article. The author would like to acknowledge CSMA Ltd. for their help with XPS and ToF-SIMS analysis, and Lori Jones of Dow Corning Corp. for her assistance with the chemistry of silicone release systems.This article is based on a paper being presented at PSTC Tech XXVI, May 12-14 in Orlando.
For more information on release liners, contact Akrosil Division of International Paper, 206 Garfield Ave., Box 8001, Menasha, WI 54952; Phone (920) 729-5309, attn: Lisa Schaaf, e-mail lisa.schaaf@ipaper.com ; or visit http://www.akrosil.com .
