Mercury-Free Polyurethane Adhesives
Three new PU hardeners are free of mercury catalysts but offer final bonding characteristics similar to traditional mercury-containing adhesives.
 “Adhesives are social substances,” writes Irving Skiest, author of Handbook of Adhesives. “They unite materials, creating a whole that is greater than the sum of parts.” Adhesives bond similar or dissimilar substrates, and growth in the adhesives industry has emanated from its advantages over other joining methods. For an adhesive to bond two substrates, it must: a) wet the substrate, b) chemically bond with the substrate, and c) have good cohesive strength. It is sometimes necessary to incorporate additives to wet the surface and bond with the substrate.
“Adhesives are social substances,” writes Irving Skiest, author of Handbook of Adhesives. “They unite materials, creating a whole that is greater than the sum of parts.” Adhesives bond similar or dissimilar substrates, and growth in the adhesives industry has emanated from its advantages over other joining methods. For an adhesive to bond two substrates, it must: a) wet the substrate, b) chemically bond with the substrate, and c) have good cohesive strength. It is sometimes necessary to incorporate additives to wet the surface and bond with the substrate.
Keep in mind that adhesion is not a property of a material; it depends on many factors, including substrates (mainly the surface, not bulk), surface preparation, adhesive type (chemistry), specimen geometry (joint design), test method and environment. In the case of bonding zinc-coated steel with aluminum, for example, it is the aluminum and zinc surfaces that have to bond, not the steel.
Due to the growing concern over the toxicity of organo-mercury compounds in the environment, there is a universal effort to replace mercury-containing polyurethane (PU) adhesives. Organo-mercury compounds are used as catalysts in PU adhesives. The PU adhesives described in this paper represent 100% solid, two-component (2K) systems.

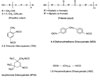 Though NCO is a highly reactive group, it shows different reactivity toward the active hydrogen, depending on the base structure. Aromatic isocyanates, TDI and MDI have been workhorses in polyurethane adhesives. However, in some special adhesives, where UV resistance is critical, aliphatic isocyanates have been used. The isocyanates are mostly difunctional, though the functionality can range from 2.0 to 2.7.
Though NCO is a highly reactive group, it shows different reactivity toward the active hydrogen, depending on the base structure. Aromatic isocyanates, TDI and MDI have been workhorses in polyurethane adhesives. However, in some special adhesives, where UV resistance is critical, aliphatic isocyanates have been used. The isocyanates are mostly difunctional, though the functionality can range from 2.0 to 2.7.
In adhesives, oligomeric (mol. wt. 300-4000) polyols are generally used. These polyols are based on either glycols (polyether polyols) or the ester condensation product of a diol and a diacid with OH end groups. The ester-type polyols are called polyester polyols. The reactivity of the OH group influences the kinetics of the PU reaction; for example, the primary OH group reacts three times faster than the secondary OH group and eight times faster than water. The general chemical structure of various polyols and diisocyanates is shown in Figure 3.
 The performance of a polyurethane adhesive depends on the structure of both polyols and isocyanates. Polyether polyols yield softer (low modulus) material, whereas polyester polyols result in a relatively rigid system. Similarly, adhesives based on aromatic isocyanates have higher modulus compared with those based on aliphatic isocyanates. The availability of a variety of polyols and diisocyanates enables the design of adhesive materials with a wide range of characteristics (e.g., from low modulus to a highly tough material).
The performance of a polyurethane adhesive depends on the structure of both polyols and isocyanates. Polyether polyols yield softer (low modulus) material, whereas polyester polyols result in a relatively rigid system. Similarly, adhesives based on aromatic isocyanates have higher modulus compared with those based on aliphatic isocyanates. The availability of a variety of polyols and diisocyanates enables the design of adhesive materials with a wide range of characteristics (e.g., from low modulus to a highly tough material).
 Catalysts play a major role in polyurethane chemistry. The most common groups of catalysts are tertiary amine-based and organometallic type (Bi, Hg, Zn, Sn, Ti, etc). Tertiary amines catalyze by activating the carbonyl carbon of the isocyanate, while organometallic catalysts work differently by complexing the incoming hydroxyl group with the isocyanate group. The details of the actual mechanism are poorly understood; however, it is believed that the oxygen of the hydroxyl group certainly attacks the carbon of the NCO group. (The role of a mercury catalyst is explained in the next section.)
Catalysts play a major role in polyurethane chemistry. The most common groups of catalysts are tertiary amine-based and organometallic type (Bi, Hg, Zn, Sn, Ti, etc). Tertiary amines catalyze by activating the carbonyl carbon of the isocyanate, while organometallic catalysts work differently by complexing the incoming hydroxyl group with the isocyanate group. The details of the actual mechanism are poorly understood; however, it is believed that the oxygen of the hydroxyl group certainly attacks the carbon of the NCO group. (The role of a mercury catalyst is explained in the next section.)

 Due to the change in the mercury-free composition, slight changes on weight basis were made. However, the mix ratio by volume was kept the same as the mercury-containing versions. The same resin (Araldite® 8680) was used for the evaluation of all three hardeners; the comparative density and viscosity data are shown in Figures 4 and 5.
Due to the change in the mercury-free composition, slight changes on weight basis were made. However, the mix ratio by volume was kept the same as the mercury-containing versions. The same resin (Araldite® 8680) was used for the evaluation of all three hardeners; the comparative density and viscosity data are shown in Figures 4 and 5.
As is evident from the data, the properties of non-mercury adhesives are similar to the previous mercury-containing adhesives. It is clear that, barring slight variations, gel time, viscosity and density of mercury-free PU adhesives match that of the mercury-containing versions, which suggests that the bulk properties remain similar. One of the challenges in this PU hardener development was to match the gel time of the mercury and non-mercury versions. As shown in Figure 6, both versions show similar cure behavior, though mercury-bearing PU hardeners exhibit a unique cure profile that will be discussed later. It is interesting to note that, although the bulk properties seem to be unaffected by the change in the catalyst, some changes occur in the early part of the curing process, as seen in Figures 7 and 8.
 Compared to the mercury-containing adhesives, it takes longer for the non-mercury versions to reach a lap shear strength (LSS) of either 145 psi or 1160 psi. The reverse seems to be the case with the Araldite® 2041 hardener and its non-mercury analog. In all three cases, the ultimate LSS reaches the same level after a 24-hour cure. This suggests that, in selective cases, the catalyst plays a role in the initial stages of the cure but has no effect on the final cure.
Compared to the mercury-containing adhesives, it takes longer for the non-mercury versions to reach a lap shear strength (LSS) of either 145 psi or 1160 psi. The reverse seems to be the case with the Araldite® 2041 hardener and its non-mercury analog. In all three cases, the ultimate LSS reaches the same level after a 24-hour cure. This suggests that, in selective cases, the catalyst plays a role in the initial stages of the cure but has no effect on the final cure.
 The effect of a catalyst on the thermal properties can be seen in Table 2. It appears that, other than the cure profile, the catalyst does not seem to affect the thermal properties of the adhesive system.
The effect of a catalyst on the thermal properties can be seen in Table 2. It appears that, other than the cure profile, the catalyst does not seem to affect the thermal properties of the adhesive system.
 In the case of PU systems, the lower Tg is controlled by the type of polyol, whereas the upper Tg is mainly influenced by the degree of crosslinking or network. The efficacy of the non-mercury catalyst replacing a mercury catalyst is also evident by the hardness values. Like the Tg, the ultimate bulk property is independent of the catalyst.
In the case of PU systems, the lower Tg is controlled by the type of polyol, whereas the upper Tg is mainly influenced by the degree of crosslinking or network. The efficacy of the non-mercury catalyst replacing a mercury catalyst is also evident by the hardness values. Like the Tg, the ultimate bulk property is independent of the catalyst.
 The cure profile of a mercury catalyst is unique, as shown in Figure 9. (Figure 9 is provided as a reference to show the profile difference between a mercury-containing catalyst and a non-mercury catalyst in the PU system. King Industries developed the PU system mentioned in the figure.) The profile looks like a “step function” (i.e., no gelation occurs for an extended period, but is then followed by quick cure). The profile provides the user with sufficient time to align or realign the joints after the adhesive is applied. Finding a catalyst that mimics the mercury profile has been challenging; to achieve that goal, formulators have tried more than one catalyst in the PU hardener formulation. The unique qualities of the mercury catalyst have been attributed to the stability of the intermediate and its planar structure.
The cure profile of a mercury catalyst is unique, as shown in Figure 9. (Figure 9 is provided as a reference to show the profile difference between a mercury-containing catalyst and a non-mercury catalyst in the PU system. King Industries developed the PU system mentioned in the figure.) The profile looks like a “step function” (i.e., no gelation occurs for an extended period, but is then followed by quick cure). The profile provides the user with sufficient time to align or realign the joints after the adhesive is applied. Finding a catalyst that mimics the mercury profile has been challenging; to achieve that goal, formulators have tried more than one catalyst in the PU hardener formulation. The unique qualities of the mercury catalyst have been attributed to the stability of the intermediate and its planar structure.
As can be seen from the data, a subtle difference exists in the initial stages of the cure (i.e., reaching the green strength of LSS > 145 psi or LSS > 1160 psi). However, no significant difference is seen in the ultimate LSS of these adhesives, with or without mercury-containing catalysts. Though green strength values do not indicate the ultimate strength of the adhesive, these values nevertheless allow the user to set the bonding part for the next stage of production.
For additional information, contact the Huntsman Advanced Technology Center, 8600 Gosling Road, The Woodlands, Texas 77381; phone (281) 719-7913; fax (281) 719-7500; e-mail hans_kaul@huntsman.com; or visit www.huntsman.com.
2. Hartshorn, S. R., Ed., Structural Adhesives, Chemistry and Technology, Plenum Press, New York, 1986.
3. The Adhesive and Sealant Council, Inc., 2005-2007 North American Market Study for Adhesives and Sealants, 2006.
4. Saunders, J. H., Frisch, K. C., Polyurethanes: Chemistry and Technology, Interscience Publishers, New York, 1962.
5. Wiltjam Abbate, F., Ulrich, Henri, “Urethanes. I. Organometallic Catalysis of the Reaction of Alcohols with Isocyanates,” Journal of Applied Polymer Science, Vol. 13, pp. 1929-1936.

Figure 1. Urethane Structure
Keep in mind that adhesion is not a property of a material; it depends on many factors, including substrates (mainly the surface, not bulk), surface preparation, adhesive type (chemistry), specimen geometry (joint design), test method and environment. In the case of bonding zinc-coated steel with aluminum, for example, it is the aluminum and zinc surfaces that have to bond, not the steel.
Due to the growing concern over the toxicity of organo-mercury compounds in the environment, there is a universal effort to replace mercury-containing polyurethane (PU) adhesives. Organo-mercury compounds are used as catalysts in PU adhesives. The PU adhesives described in this paper represent 100% solid, two-component (2K) systems.

Figure 2. Polyurethane Structure
Polyurethane Systems
In general, a urethane bond is formed when a hydroxyl group reacts with an isocyanate group (see Figure 1). Similarly, a reaction of a diol with a diisocyanate gives rise to a polyurethane structure, as shown in Figure 2. The reactions of diisocyanates are usually more complicated than those of monoisocyanates. The initial reactivity of a diisocyanate is similar to that of a monoisocyanate substituted by an activating group (in this case, another NCO group). In the case of aliphatic diisocyanates (isophorone diisocyanate, IPDI) or aromatic diisocyanates (toluene diisocyanate, TDI; or methylene diphenyl diisocyanate, MDI), where the phenyl rings are separated by one or more CH2 groups, one NCO group has a lesser effect on the other. Nevertheless, the minor reactivity difference still exists.
Figure 3. Structures of Various Polyols (Diols) and Diisocyanates
In adhesives, oligomeric (mol. wt. 300-4000) polyols are generally used. These polyols are based on either glycols (polyether polyols) or the ester condensation product of a diol and a diacid with OH end groups. The ester-type polyols are called polyester polyols. The reactivity of the OH group influences the kinetics of the PU reaction; for example, the primary OH group reacts three times faster than the secondary OH group and eight times faster than water. The general chemical structure of various polyols and diisocyanates is shown in Figure 3.

Figure 4. Density Data of PU Adhesive Hardeners

Figure 5. Viscosity Data of PU Adhesive Hardeners

New PU Adhesives
Huntsman Advanced Materials has developed three new PU hardeners that are free of mercury catalysts. (RD2008-017 is a replacement for the commercial Araldite® 2041 hardener, RD2008-050 is a replacement for Araldite® 2042, and RD2008-053 replaces Araldite® 8685.) All three hardeners are formulated compositions. The mix ratio of these adhesives, by weight and by volume, is shown in Table 1.
Figure 6. Gel Time Data of PU Adhesives
As is evident from the data, the properties of non-mercury adhesives are similar to the previous mercury-containing adhesives. It is clear that, barring slight variations, gel time, viscosity and density of mercury-free PU adhesives match that of the mercury-containing versions, which suggests that the bulk properties remain similar. One of the challenges in this PU hardener development was to match the gel time of the mercury and non-mercury versions. As shown in Figure 6, both versions show similar cure behavior, though mercury-bearing PU hardeners exhibit a unique cure profile that will be discussed later. It is interesting to note that, although the bulk properties seem to be unaffected by the change in the catalyst, some changes occur in the early part of the curing process, as seen in Figures 7 and 8.

Figure 7. Cure Time to Reach LSS of 145 psi

Figure 8. Cure Time to Reach LSS of 1160 psi


Figure 9. Cure Profile of Mercury and Non-Mercury Catalyst in PU Systems
As can be seen from the data, a subtle difference exists in the initial stages of the cure (i.e., reaching the green strength of LSS > 145 psi or LSS > 1160 psi). However, no significant difference is seen in the ultimate LSS of these adhesives, with or without mercury-containing catalysts. Though green strength values do not indicate the ultimate strength of the adhesive, these values nevertheless allow the user to set the bonding part for the next stage of production.
Safer Alternatives
Three new mercury-free adhesives have been developed to replace previously mercury-containing versions. The bulk properties and the ultimate performance of mercury and non-mercury versions are similar. The new safer alternatives enable users to easily switch from the mercury version to the non-mercury version without worrying about a loss of performance in the final bonding.For additional information, contact the Huntsman Advanced Technology Center, 8600 Gosling Road, The Woodlands, Texas 77381; phone (281) 719-7913; fax (281) 719-7500; e-mail hans_kaul@huntsman.com; or visit www.huntsman.com.
For Further Reading
1. Irving, Skiest, Ed., Handbook of Adhesives, Third Edition, Van Nostrand Rheinhold, 1990.2. Hartshorn, S. R., Ed., Structural Adhesives, Chemistry and Technology, Plenum Press, New York, 1986.
3. The Adhesive and Sealant Council, Inc., 2005-2007 North American Market Study for Adhesives and Sealants, 2006.
4. Saunders, J. H., Frisch, K. C., Polyurethanes: Chemistry and Technology, Interscience Publishers, New York, 1962.
5. Wiltjam Abbate, F., Ulrich, Henri, “Urethanes. I. Organometallic Catalysis of the Reaction of Alcohols with Isocyanates,” Journal of Applied Polymer Science, Vol. 13, pp. 1929-1936.
Authors' Acknowledgements
First, we would like to thank Ash Chaudhari; K. P. Subhramanian; Derek Kincaid, Huntsman Advanced Materials technical management; and Doug Ellerbusch, Marketing manager, for their support and encouragement. We would also like to thank Lorenzo Petway and Alan Hamilton for their help in reviewing this paper. Our thanks also go to Richard Shain with King Industries for permitting us to incorporate the cure profile of mercury and non-mercury catalysts developed by King Industries. Finally, we thank King Industries for sending catalyst samples for evaluation.Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






