Bio-Based Feedstocks for Adhesives and Sealants: Everything Old is New Again
Recent years have seen a near adversarial relationship between bio-based product manufacturers and petrochemical companies.

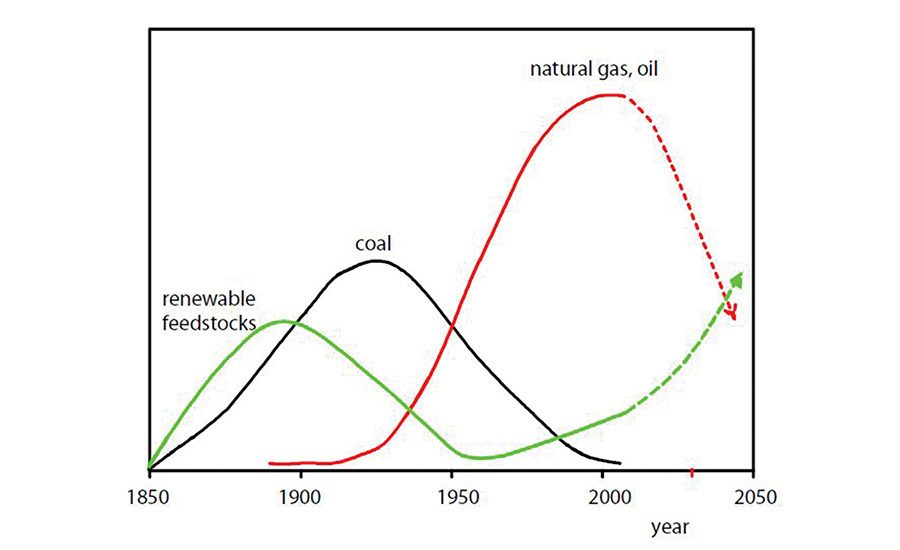








“Don’t throw the past away
You might need it some rainy day
Dreams can come true again
When everything old is new again”
— Everything Old is New Again, Peter Allen
This is a call for innovation and collaboration. The ascent—or, more appropriately—re-ascent of renewable chemistry provides great promise for the adhesives and sealants industry. However, the full promise of these innovations can only be commercially realized through collaboration between renewable innovators, synthetic chemistry providers, and downstream formulators.
Recent years have seen a near adversarial relationship between bio-based product manufacturers and petrochemical companies. Why is the status quo for raw materials in the chemical industry in its current state?
Bio-Based Basics
Bio-based materials are marketed as novel building blocks that are made and used more sustainably than traditional petrochemical-based alternatives. Independent certifications continue to proliferate, allowing consumer product manufacturers to highlight their efforts to improve energy efficiency, reduce carbon dioxide emissions and accentuate responsible raw material sourcing on product labels.
The field of lifecycle analysis (LCA) has blossomed in recent years, providing quantitative methods for companies to compare the relative environmental impact of one formulated product or method of production to that of a competitor. The potential benefits of being able to market a product as “green,” “low-VOC,” “renewable” or “sustainable” are often outweighed by the perceived higher cost of using bio-based materials. Where does this perception come from? Is it real?
It is true that some bio-based building blocks are so different from their petrochemical analogs that reformulation is required to match the same performance qualities of the original product. During this process, cosmetic properties such as smell or color can change. While these attributes may seem trivial, they are often crucial to the final consumer or end user’s satisfaction.
Many household cleaners are either pine or lemon scented, which consumers associate with cleanliness. Other sensory experiences generate specific and unique consumer reactions. Formulators and brand managers know this. For the last 60-70 years, most of these formulated products were delivered through the promise of synthetic chemistry. But what was used before petrochemicals? How did the adhesives and sealants industry account for the change to petrochemicals?
Adhesives in History
The first adhesives and sealants were formulated nearly 200,000 years ago, long before the advent of modern petrochemicals. In the Pleistocene era, it is believed that axes were constructed using a tar derived from tree bark. Archeological findings show that early humans understood the ingredients that the world around them offered, and exploited them to the best of their knowledge.
For example, 70,000 years ago in South Africa, it is believed that a two-component adhesive was used for the first time. This formulation was used to create tools that were more resistant to water exposure than previous one-component systems. The use of this two-component formulation (plant resin and iron oxide) is historically important as it signals the use of a rationally designed adhesive and hence, arguably, the start of the adhesives and sealants industry.
Ingredients that we now call “natural” or “renewable” were the exclusive materials in the adhesive and sealants chemists’ toolboxes for tens of thousands of years. Notable events in the history of adhesives, all of which involve the use of naturally derived ingredients, include a casket glued together in the tomb of King Tut (circa 1323 B.C.); the creation of wood veneers and wax-sealed ships by the Romans (circa 27 B.C.); the first glue plant opened in Holland using animal hides (1690 A.D.); and the first U.S. postage stamps, which debuted in 1876 using starch-based adhesives.
Even cellophane, a near-ubiquitous packaging material, is made from regenerated cellulose, the most abundant biomaterial on the planet. The adhesives and sealants industry was built upon ingredients that are very unlike the petrochemical ones that are chiefly used today. Casein glue, as well as adhesives based on starch, cellulose, and natural rubber, have all been staples of the adhesives and sealants industry—and are all bio-based. So how did this industry (and others) change from the near-exclusive use of bio-based materials to largely relying on petrochemicals and their derivatives?
Two words: cheap oil. While the long-term scarcity and environmental impact of fossil-based resources is well-understood, the benefits of petrochemicals are without question. Modern synthetic chemistry has brought tremendous advances to humankind and will continue to provide innovations well into the future. To many in the chemical industry, bio-based materials are a fad built upon the hype of environmentalists, non-government organizations (NGOs) and mass media. Bio-skeptics believe that as these concerns quiet with time, consumer demand for bio-materials will fade away. However, one must carefully consider this perspective. Is there any value offered by bio-materials that is not paralleled by petrochemicals?
Like any scientist, formulators in this industry and others have valid concerns about changing their raw material sourcing. These concerns are largely driven by the risk of changing non-functional properties that customers have grown accustomed to and now demand. In reality, formulators previously conquered this burden when they added petrochemicals to their toolbox. In fact, it is indisputable that the addition of petrochemicals, with their diverse structures and reactivities, greatly enriched the quality of products produced by the chemical industry. Inventions such as cyanoacrylate, the key component of superglue, would not have been possible at the time of their invention (1940s) without cheap raw materials and the functional diversity afforded to petrochemicals. However, it was known when the great oil age began that the great promise of petrochemicals had an expiration date. Without question, it has been well-understood from the advent of cheap oil in the 1930s that oil was (and is) a finite resource.
On a relative scale, oil and petrochemicals may very well have a temporary existence in the toolboxes of formulators in all chemical industries. This article serves as an encouraging wakeup call that builds upon the insight of the petrochemical industry and its rich history of innovation. The chief concerns that formulators and brand managers have with bio-based materials (e.g., difficulty of formulation, dealing with impurities and consumer sensory experiences) were also concerns when chemical industries switched from naturally sourced raw materials to petrochemicals. There is nothing special about oil’s potential for diversely functionalized materials, nor are bio-materials blessed with a similar “magic.” Scientists not only adapted to petroleum when it was the new raw material source, but also harnessed its potential and innovated like the world has never seen before.
Better Chemical Sourcing
No one believes the sky is falling, nor does anyone long for the last drop of oil to be pulled out of the earth. The truth is quite honestly the opposite. Chemical industries manipulate matter for the benefit of mankind. As such, should scientists not look everywhere for sources of this matter to manipulate? Is anyone better-suited than the present chemical industry to push bio-based materials to their limit and extract all their potential functionality? What happens when the modern chemical industry embraces a biotechnology method and encourages its growth?
What about industrial ethanol? It is a little-known fact that Henry Ford’s Model T could be modified to run on either gasoline or pure ethanol. Standard Oil even sold a gasoline blend that contained 25% ethanol. At the time, all ethanol was produced through fermentation of agriculture with yeast as the biocatalyst. In the post-war era of the 1940s, however, all fuel efforts shifted to the widely available and cheap high-energy compounds that could be distilled from crude oil. This continued unabated until the late 1970s, when the confluence of tight oil supply (due to turmoil in the Middle East) and regulatory issues (including mandates for the removal of lead from gasoline) forced a change.
Due to the rich history and proven technology of using agriculturally derived ethanol as an automotive fuel, industry and Congress focused on this simple two-carbon alcohol. While not as high in energy content as traditional gasoline, ethanol is an adequate fuel. In addition, it is capable of boosting the octane rating in gasoline blends without the addition of a toxic substance such as lead.
Subsidies at both the federal and state level caused the price of agriculturally derived ethanol to be competitive with gasoline refined from oil. In the one-year period between 1979 and 1980, ethanol production in the U.S. jumped from 10 million gal to 175 million gal. In 2014, the monthly average demand for ethanol was over 14 billion gal. During this same time, the ratio of agriculturally derived ethanol to synthetic ethanol drastically changed. In 1980, greater than 95% of ethanol in the U.S was produced by hydration of ethylene. Today, synthetic processes account for less than 20% of the ethanol consumed in the U.S. Agriculturally derived (or “natural”) ethanol makes up the rest.
Agriculturally derived ethanol became profitable to a large degree because of federal and state subsidies. However, it would be foolish to think that 40 years of a cunning and innovative petrochemical industry did not play a large role in the efficient design and operation of ethanol fermentation plants. In fact, innovation in ethanol production was driven by chemical producers, as the ethanol that was being produced in these agricultural fermentation facilities was blended in with petrochemically refined gasoline; this continues today. The ethanol story is a world-scale example of the incredible success than can result from collaboration between the petrochemical industry and biotechnology. What else is possible?
Ethanol is one of the world’s oldest industrial chemicals; its modern fermentation-based production for use in gasoline blends is a true success story of the marriage between the petrochemical industry and biotechnology. Its success is largely due to the fact that the methods to produce ethanol were discovered long ago, and modern engineering simply improved the process. These process improvements, along with strong historical knowledge and a fair bit of government intervention, are the reasons that all the cars that run on gasoline in the U.S can run on an ethanol/gasoline fuel blend. There is only one process similar to ethanol in its historical story and potential for modern success: ABE fermentation.
ABE, or acetone, n-butanol, ethanol (ABE) fermentations were technically first performed in 1861 by Louis Pasteur, but the technology had to wait for half of a century before it was industrially viable. In 1912, while researching biotechnological methods of producing rubber in the UK, Chaim Weizmann, Ph.D., identified a specific bacterium, Clostridia acetobutylicium, capable of producing acetone, n-butanol, and ethanol in a 3:6:1 ratio from the fermentation of starches and sugars. Although Weizmann’s goal of a bio-based rubber was not achieved, he began patenting his methods for the industrialization of ABE fermentations around the world in 1916. The solvents produced by Weizmann’s ABE technology proved to be immensely valuable in years that followed.
At that time, acetone was primarily produced through the dry distillation of acetates (i.e., calcium diacetate) and was far below the purity that chemists are accustomed to today. World War I broke out in 1914 and, as it was primarily fought through trench warfare, the Allies relied on cordite, a type of smokeless gun powder, to fire their artillery without being detected. Yet during the war, large amounts of acetone were needed and the dry distillation of metallic acetates produced very low purity acetone, especially at large scale. As a result, the cordite produced using this acetone failed to be completely smokeless. However, the acetone produced through ABE fermentations was extremely pure, as it was directly distilled from a fermentation broth containing minimal volatile organics and free from thermally decomposed material. When this came to the attention of the manufacturers of cordite, Weizmann’s acetone was exclusively used throughout the entire war effort for cordite produced in the UK.
To many, Weizmann was a hero of WWI for England and the rest of the Allies. ABE fermentation facilities began to spring up all around the world, with Weizmann’s company, Commercial Solvents Corp., opening its largest facility in Peoria, Ill. While the technology came to the world’s attention due to its ability to produce high-purity acetone, the co-product n-butanol soon became the star of the show, though its utility was not immediately realized. In fact, many facilities run by Commercial Solvents simply ran their plants to produce acetone, and stored the n-butanol in gigantic storage vessels.
As the n-butanol began dominating the storage of these facilities (twice as much n-butanol is produced than acetone in ABE fermentations), scientists quickly found uses for it. First, n-butanol is a tremendously useful fuel. It has 98% of the energy density of gasoline (compared to 67% with ethanol) and can either be used exclusively in traditional internal combustion engines or in blends (up to 18%) with gasoline. n-butanol itself was used to fuel aircraft for many of the vehicles in the British Royal Air Force during WWII due to the security of supply and its incredibly purity (for largely the same reasons as acetone, distillation from a fermentation broth is cleaner than from metallic acetates or petrochemicals).
As an alcohol, n-butanol has a fair degree of reactivity and can be chemically transformed into a variety of derivatives. n-butyl acetate, an ester of n-butanol and acetic acid, was developed as a favorite solvent of paints and coatings companies in the 1930s and 1940s for use in dissolving nitrocellulose compounds. Lacquers produced with n-butyl acetate as the solvent were more robust than any that had been previously made. This revolution resulted in car paints that lasted years, when previously paint jobs had lasted months. n-butyl acrylate, an ester of acrylic acid and n-butanol, also was shown to be an incredibly valuable derivative. This molecule, which bears a a,b-unsaturated moiety, is still used prolifically in polymers and resins throughout the adhesive and sealants industry, as well in consumer paints and nail polishes.
The utility of high-purity acetone, n-butanol and derivatives of these compounds led to research into all types of methods for improving fermentation yields, as well as alternative methods of production. As the oil boom provided the world with cheap crude petroleum and petroleum distillates, elucidation of the various types of molecules able to be isolated was hot research. One such compound was propylene. This three-carbon olefin was shown to be incredibly versatile, as it could be polymerized for plastics (polypropylene) or transformed into higher value derivatives. Both acetone and n-butanol could be made from propylene, albeit through different methods.
Acetone can be made using propylene and benzene in what is referred to as the cumene process. Phenol, a co-product of the synthesis, is more highly valued than acetone and is a large driver of the economics of the process. While this helps to keep acetone prices relatively low, acetone produced using this method can contain traces of the benzene starting material or the co-product phenol, even after distillation. Both of these molecules are toxic aromatic species and as such, acetone, a generally safe molecule that is produced during normal mammalian metabolism, must be tested for trace contamination by these compounds.
n-butanol is also produced from propylene through what is called the oxo-process, which is a two-step process consisting of hydroformulation and reduction. Propylene is exposed to carbon monoxide and hydrogen gases under high pressure and metal catalysis (initially cobalt, but rhodium since the 1960s) to form a mixture of isobutyraldehyde and n-butyraldehyde. The ratio of these two co-products can be tweaked by varying the ligands used with the catalyst. The mixture of butyraldehydes is then reduced using more hydrogen and metal catalysis to produce isobutanol and n-butanol, which are then purified by distillation. As with acetone produced from petrochemicals, n-butanol can contain traces of its intermediates, the butyraldehydes, or co-product, isobutanol.
Acetone and n-butanol produced through the ABE fermentation methods are intrinsically higher purity than those made from petrochemicals. As previously mentioned, ABE-produced acetone contains no aromatics such as benzene and phenol, and ABE-produced n-butanol does not contain aldehydes or isobutanol. Despite these benefits, both butanol and acetone were much cheaper to produce through processing of propylene, and fermentation processes could no longer compete. ABE production shut down in North America in the 1950s, and the final traditional ABE plants closed in South Africa in the 1980s.
As petrochemicals displaced fermentation, formulators conquered the burden of dealing with a new supply of raw materials that contained new trace impurities. All products that had been made using the ABE produced acetone or n-butanol had to account for the use of the petrochemical versions of these compounds and the molecules that came along with them. This all was done with the technology available to chemists in the 1940s and ’50s. As this example shows, formulators are excellent, resourceful innovators. In adhesives and sealants and all other chemical industries, formulators are ready and capable to produce better, more useful products no matter the raw material source.
ABE fermentations are not new. In fact, these bacterial fermentations are directly analogous to the ethanol yeast fermentations that have been used for millennia to produce alcoholic beverages. In addition, the products of these fermentations, n-butanol and acetone, are molecularly identical to the compounds produced through petrochemical methods. Yet since the ABE process does not have volatile organic inputs, and produces minimal such compounds other than acetone, n-butanol, and ethanol, it is intrinsically free from the common impurities that come with petrochemically produced versions of these molecules. Advanced fermentation protocols, cutting-edge separation technology, and a robust library of diverse organisms capable of producing solvents at varying rates and by using diverse and cheap sources of sugar are key for an ABE fermentation venture to succeed.
Reintegrating ABE Technology
Presently, a few companies are still pursuing the goal of reintegrating ABE technology into the chemical industry. Alphabetically, the leading companies include Celtic Renewables, Cobalt Technologies, and Green Biologics Ltd. Celtic Renewables is a Scottish company focused on using whiskey manufacturing residues as a feedstock, while Cobalt Technologies is focused on using cellulosic agricultural residues as a feedstock. Green Biologics is focused on improving the ABE process to allow for more economical separation of the solvents from the fermentation broth. Green Biologics has acquired a 21 MGPY fermentation production facility in Little Falls, Minn., and plans to commercially introduce renewable n-butanol and acetone in 2016.
Collaboration for Innovation
ABE fermentations are just one example of history repeating itself, where what was old is new again. The future of sustainability and innovation does not lie with those that seek to compete with the giants of the petrochemical industry. Bio-based materials companies need collaborations with petrochemical producers. Chemical producers and formulators need the innovation and advances of biotechnology.
Advanced microbiology and state-of-the-art engineering can make most biotech ideas possible, but recent history has shown us that without collaborations with the petrochemical industry, they are seldom commercially viable. After all, petrochemical formulators conquered the hurdle of switching from bio-based materials to petrochemicals in the 1940s. Who else is better-suited to teach us all how to make the journey back again?
For more information, contact the author at (804) 368-6136 or lee.speight@greenbiologics.com, or visit www.greenbiologics.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!













