Food safety matters continue to take center stage across the entire supply chain. Over the last two decades, a series of high-profile international food safety concerns—from bovine spongiform encephalopathy (BSE), listeria, dioxin and other toxins—have given rise to industry-driven initiatives such as the Global Food Safety Initiative (GFSI) and other legislative actions around the world. The goals of GFSI have been to reduce risk throughout the food supply chain, implement consistent standards globally and restore confidence in the safety of the food delivered to consumers worldwide.
More recently, the food safety concern has extended beyond cases of intentional and unintentional contamination of the food itself to migration of materials from packaging, such as additives from ink, coatings and adhesives into the food. Standards and procedures to evaluate and address migration vary, and the EU in particular is reevaluating this issue. For example, EU adhesive producers are concerned with plasticizer migration.1,6,9
Current EU regulations regarding packaging additives and migration cover plastics rather than adhesives (EC 10/2011);2 however, they are routinely used as best-practice models for all food packaging materials, including adhesives. In the U.S., the specific Food and Drug Administration (FDA) regulation for adhesive use in food packaging is 21 CFR 175.105,7 which assumes that the only adhesive-food contact would be at a thin glue line used to seal the packaging. However, many adhesive producers also seek to meet the requirements for 21 CFR 176.170,8 which concerns coatings on paperboard and paperboard components that come in direct contact with fatty and aqueous food, and for 21 CFR 176.180, a regulation addressing coatings in contact with dry food. Whether in the U.S. or EU, different approaches can be taken to meet both current regulations and manufacturers’ safety objectives for migration of additives, such as plasticizers.
Copolymer producers do offer plasticizer-free solutions; copolymers are certainly soft enough without a plasticizer.3,4,5 However, plasticizers are not only useful for their ability to soften; they also allow formulators the versatility to use homopolymer polyvinyl acetate and optimize either polymeric system for desirable performance characteristics including increased viscosity (viscosity response), increased open time, reduced set times, and tackiness. A number of solutions are available today that allow formulators to achieve the versatility and performance desired in an adhesive, while also meeting best practices for food safety and migration.
Background
In general, there is a need for new products for this industry to address new applications and upgrade performance. Needs develop as performance or regulatory environments change. This is true for other segments, as well as adhesives. For example, the vinyl industry has contended with regulatory issues surrounding phthalate use, causing a significant shift from phthalates to “non-phthalates.” That shift created new opportunities for development. However, new technologies for food packaging face barriers in the EU, as they not only need to meet all of the required REACH registrations, but also address the significant concerns over migratories.
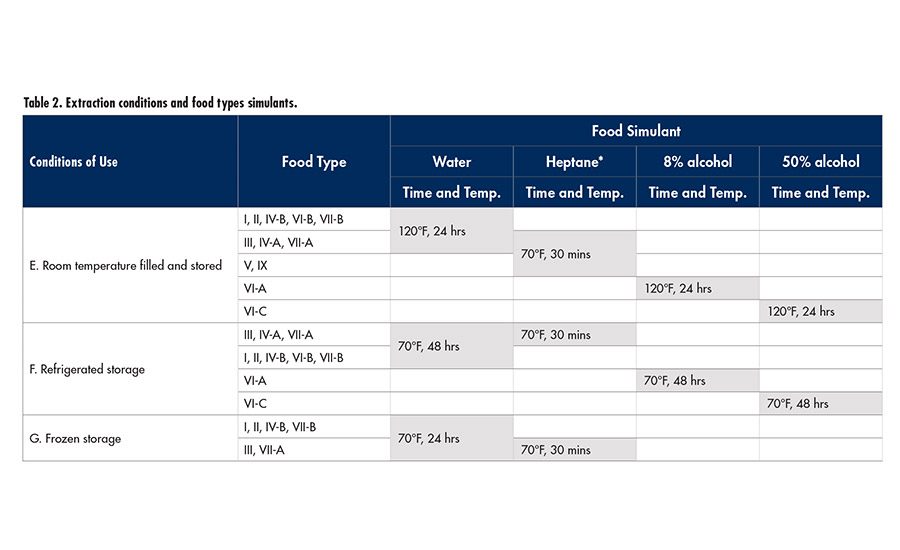
In Europe, EC 10/2011* sets limits for applications and use conditions concerning plastics used in food packaging extraction methods; test conditions are also described. While this regulation is not designed for adhesives used in the packaging industry, many adhesive manufacturers still desire that coverage.
In truth, the regulatory environment and marketplace needs are not very different in the U.S. If a chemical is new, it still needs to be listed with the U.S. Environmental Protection Agency (EPA) to get on the Toxic Substances Control Act (TSCA) list, and that is not easy. But even for existing plasticizers, certain regulatory hurdles still exist. Unlike the EU, the U.S. has well-defined FDA coverage requirements for adhesives used in packaging. Specifically, an additive such as a plasticizer must be listed in the previously mentioned 21 CFR 175.105 (covers adhesives used in packaging where the contact is only through a glue line). However, some adhesive manufacturers also require 21 CFR 176.170, which covers* coatings on paper and paperboard that come in direct contact with food. The key word here is “coating,” not “adhesive,” but this coverage does impart an additional layer of safety for adhesive additives.
A new plasticizer blend of three dibenzoates was developed for the adhesive industry. Fortunately, all the components are listed in 21 CFR 175.105, but only two are listed under 21 CFR 176.170. Prior to the investment required to conduct the full extraction evaluation required to file a Food Contact Notification (FCN), a feasibility study was conducted on extraction of the third component in line with the level of its use in the blend.
Experimental
The FDA changed how filing is completed and what needs to be considered as part of that process, but some of the tests are similar to how they used to be run. As indicated previously, plasticizers used in food packaging adhesives must have the 21 CFR 175.105 coverage. There are requirements for this coverage, but those do not include extraction; exposure through a glue line is all that is expected. Since some food packaging adhesive producers also want to meet the requirements for 21 CFR 176.170 and 21 CFR 176.180, it is important that the raw material supplier knows exactly what coverage is desired. That depends on formulation, level of plasticizer use, food types and use conditions.
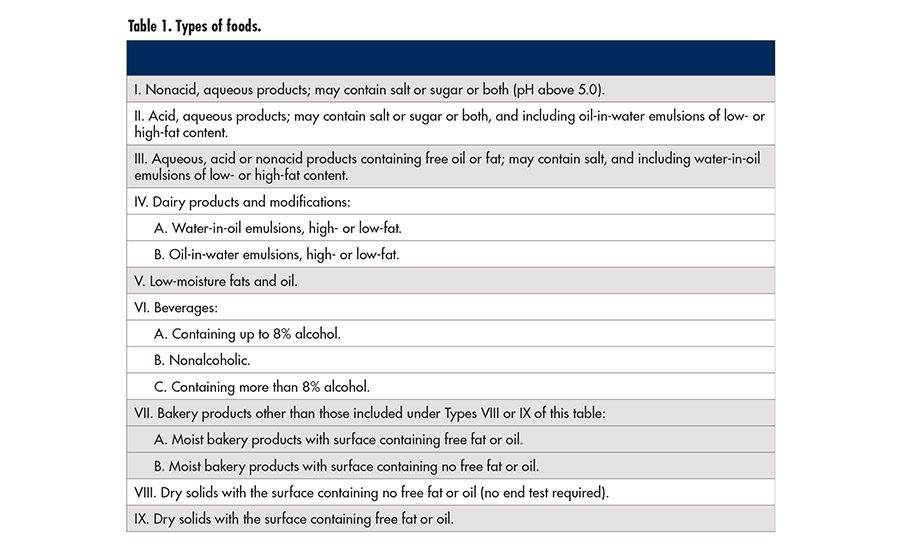
Table 1 (p. 23) shows the food types considered and the corresponding test conditions. It is crucial to understand what the conditions of use requirements need to be in order to properly design the protocol to handle extraction testing.
Based on the extraction results, a calculation of the Estimated Daily Intake (EDI, based on an average adult of 150 lbs) can be performed. The following tiered approach on required toxicology is used, which depends on coverage desired if the extracted additive EDI is:
• 0.5 50 ppb— need AMES, mouse lymphoma/chromosomal aberration assay, etc.
• 50 ppb— much more robust safety data required at this level (90-day feeding studies)
• > 1 ppm— two-year bioassay required
Any safety data that has been performed must be submitted to the FDA, even if only < 0.5 ppb exposure is expected. Additional factors related to the expected use need to be considered in these calculations. Once this information is collected, an FCN can be filed with the FDA. (The actual calculations on EDI and the other required steps will not be discussed here, as it is beyond the scope of this work. The scope is just to determine feasibility, along with the knowledge of other pieces of the puzzle needed to move forward.)
The plasticizer of focus is propylene glycol dibenzoate (K-FLEX PG). When blended with two traditional dibenzoates, it is part of a product called K-FLEX 975P. PG has 21 CFR 175.105, but not 21 CFR 176.170. The other plasticizers in the blend are dipropylene glycol dibenzoate (DP) and diethylene glycol dibenzoate (DE). Each of these plasticizers has 21 CFR 176.170, with some limitations listed.
The extraction testing protocol for DP and DE was developed several years ago, and the petitions for listing these two plasticizers were prepared for and approved by the FDA. For the current feasibility evaluation, the plan was to use the same testing methodology originally used to develop the data on DP and DE to see if it is feasible that PG could be used in combination with the DP and DE for 21 CFR 176.170 coverage on the 975P blend.
For this feasibility study, commercial-grade polyvinyl acetate homopolymer (PVA, polyvinyl alcohol protected) and vinyl acetate-ethylene copolymer (VAE, 0°C Tg, polyvinyl alcohol protected) were selected. In the PVA, the PG plasticizer loading was evaluated at 2% by dry weight; the plasticizer loading in the EVA based on wet weight was evaluated at 2%. Blank adhesive emulsions were also included for a total of four formulations.
Samples were prepared using a mixer and then aged for seven days prior to testing. Adhesive was applied to cold-rolled, annealed stainless steel at a specified film thickness, dried, and then exposed to food simulant extraction. Gravimetric and gas chromatographic analytical methods were used to measure the total migratories lost from the film. The extraction times and temperatures representing several food simulant conditions were: heptane (30 min, 70ºF), water (24 hours, 120ºF), 8% ethanol/water (24 hours, 120ºF) and 50% ethanol/water (24 hours, 120ºF).
In addition to the determination of weight lost after extraction, the amount of plasticizers left from the extraction testing in the extractibles’ residues was also determined. This was done by total extraction of remaining plasticizer in the residues by GCs of the extracts.
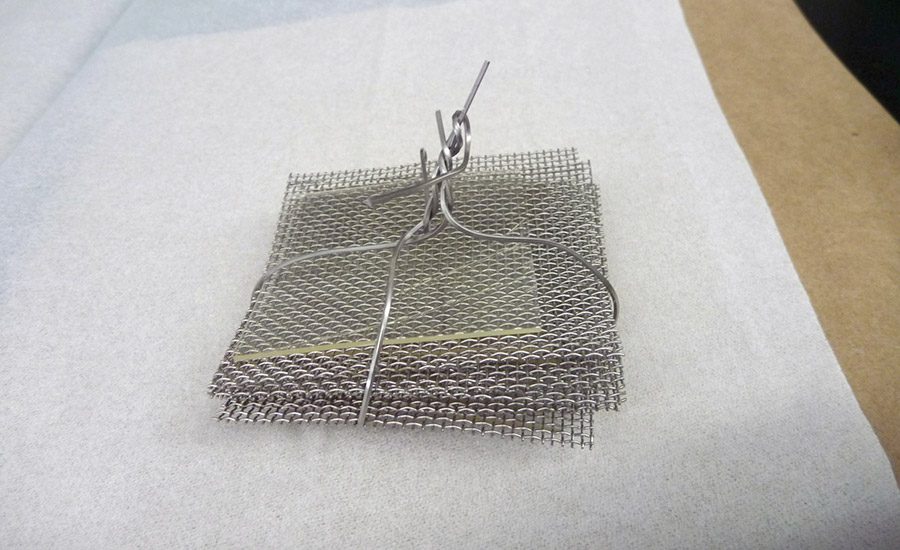
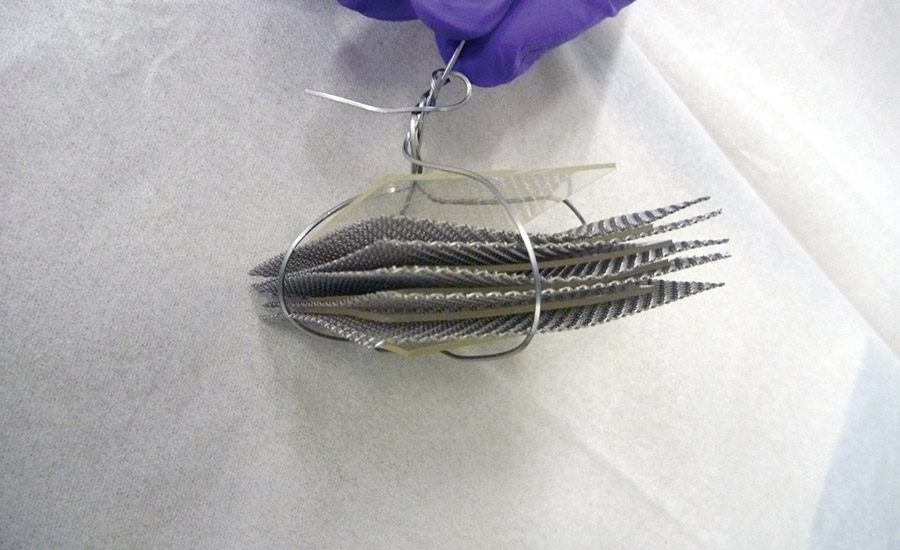
To get the proper film to conduct the extraction testing of the required 100 sq in., the adhesive-coated steel foil was cut into 2 x 2-in. squares, and a sandwich of the squares was assembled by alternating the coated squares with a mesh screen. This construct was then fastened to form the extraction “sandwich.” Figures 1-3 (p. 24) shows the process. These sandwiches were placed into the extraction media.
Results
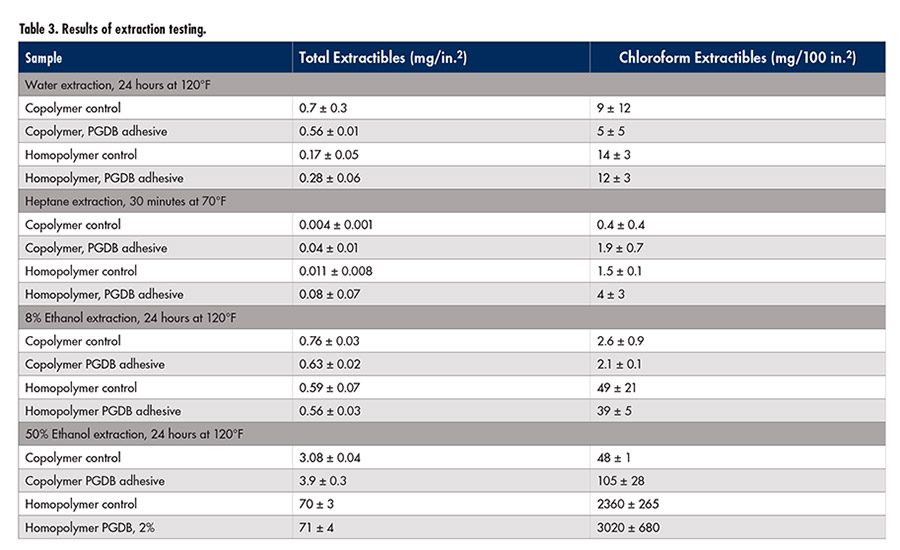
The results of the testing are listed in
Table 3 (p. 27). For the water, heptane and 8% ethanol tests, the films held up reasonably well, and little to no plasticizer extraction was noted. The amount of plasticizer in the residues from extraction testing was found to be minimal in each case, except for the 50% ethanol solutions. For the 50% ethanol test, the adhesive films experienced major breakdown, as they are not formulated to withstand exposure to such an environment. It would thus be preferable to use coating emulsions designed for such an environment rather than an adhesive designed only to have exposure at seams; this would give more realistic results.
Conclusion and Recommendation
The results are promising so far and indicate that PGDB has a reasonable opportunity to have a low enough level of extraction, especially in a properly formulated coating polymer. These are worse-case results, so it is likely that there would be even less extraction under other test conditions such as frozen storage testing protocols or with some food groups dropped.
In addition, quantification using high-pressure liquid chromatic analysis or some other means that could better quantify the extracted polymer components in addition to the plasticizers would help in determining exactly how much of the extracted material is plasticizer as compared to the starting polymer. Based on this work, it is quite likely a full screen will be considered for use in a FCN.
For more information, visit www.emeraldmaterials.com.
Acknowledgments
The authors would like to thank Ed Gotch, CEO, and Shamsi Gravel, vice president of the K-FLEX® products business of Emerald Kalama Chemical LLC, for permission to publish this data. Also, we would also like to thank the Applications Research Group for the preparation of the data.
Editor’s note: This article is based on a presentation given at the Adhesive and Sealant Council’s Spring 2015 Convention and Expo.
Disclaimer
The information contained herein is believed to be reliable, but is based on laboratory work with small-scale equipment and does not necessarily indicate end-product performance. Because of variations in methods, conditions and equipment used commercially in processing these materials, Emerald makes no representations, warranties or guarantees, express or implied, as to the suitability of the products for particular applications, including those disclosed, or the results to be obtained. Full-scale testing and end-product performance are the responsibility of the user. Emerald Performance Materials shall not be liable for and the customer assumes all risk and liability for use and handling of any materials beyond Emerald’s direct control. Nothing contained herein is to be considered as permission, recommendation nor as inducement to practice any patented invention without permission of the patent owner.
References
1. Roth, M., Momper, B. “Migration studies of adhesives based on vinyl acetate ethylene (VAE) copolymers intended for the food packaging industry.” World Adhesives Conference (WAC). Paris. 2012.2, European Union Commission Regulation No. 10/2011. Plastic materials and articles intended to come into contact with food.
2. Horwat, David and Koegler. “The Next Generation Vinyl Acetate Ethylene Copolymer Dispersions for Paper and Packaging Adhesives.” World Adhesives Conference. Miami. 2008.
3. Laugerette, Thierry. “Spotlight on Adhesives: Migration from Food Packaging.” ASI, March 2011, Pages 12-15.
4. Tönnießen, M. “Working together on safe adhesives in food packaging.” FEICA European Adhesive & Sealant Conference and Expo. Berlin, 2014.
5. FEICA, Association of the European Adhesive and Sealant Industry. Guidance for a food contact status declaration for adhesives. Brussels, 2014.
6. Code of Federal Regulations, Revised as of April 1, 2014, CITE: 21CFR176.170 Food and Drug Administration, HHS. 21 CFR PART 175.105 Indirect Food Additives: Adhesives and Components of Coatings (Section 175.105 Adhesives, Section 175.125 Pressure-sensitive adhesives).
7. Food and Drug Administration, HHS. 21 CFR PART 176 (Section 176.170 Components of paper and paperboard in contact with aqueous and fatty foods, Section 176.180 Components of paper and paperboard in contact with dry food).
8. Van Halteren, Ansgar. “Migresives: Research Programme on Migration from Adhesives into Food Packaging Materials in Support of European Legislation and Standardisation.” Industrieverband Klebstoffe e.V. WAC 2008.