I work in the technical department developing coatings based on various polymer backbones. I am familiar with typical polyurethane raw materials, but have recently become aware of polyaspartic coatings. Could you provide an overview of this technology?
The polyaspartic coatings technology has been available since the 1990s and is an extension of polyurethane chemistry. It is based on aliphatic polyisocyanates and a specialized type of co-reactant: polyaspartic esters, which are amine functional. These two-component systems are known for fast curing speeds, leading to significant increases in productivity. They are used in a range of applications, including metal coatings in bridge beams, rail cars, sports stadiums, offshore marine applications, and a variety of concrete coatings.
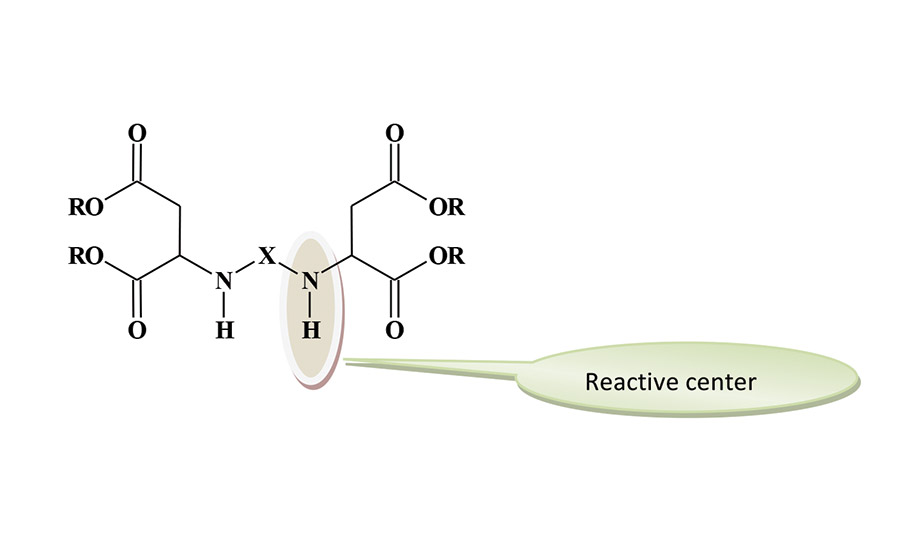
The productivity increases are not based on fast curing speeds alone. This technology has permitted for some basic changes as to how the finished coating is produced. Polyaspartic technology has made it possible in some instances to move from a conventional three-layered paint system (zinc-rich primer/epoxy/polyurethane topcoat) to a two-layer system consisting of the zinc-rich primer/polyaspartic topcoat. This has been achieved in the highly demanding corrosion protection on steel application. Direct-to-metal (DTM) coating applications are also possible with polyaspartics, and productivity is improved with the elimination of a primer step. The DTM process produced a 30% reduction in labor time in a railcar application. A generic structure for the polyaspartic ester is shown in Figure 1.
The reactive groups are the secondary amines. The sterically hindered environment of these groups reduces their reactivity compared to the reaction of typical secondary amines with polyisocyanates. The overall reactivity of the aspartates can be adjusted by changing the “X” group to influence the degree of steric hindrance. In general, the reactivity falls between the very fast curing of a polyurea polymer and the slower reaction rate of a typical polyurethane. The available grades of polyaspartic esters were designed to produce different reaction rates and can be blended to achieve the desired curing profile. The degree of steric hindrance has a limited influence on the film properties of the coating film.
The curing rate of a polyaspartic ester coating is strongly influenced by environmental conditions. As with most systems, the curing rate increases with temperature, but in this instance the rate is also greatly accelerated with increasing humidity. The second generation of polyaspartic ester technology produces formulations that are more robust and show more consistent curing times over a range of humidity levels.
Polyaspartic ester coatings formulations are unique in that they cure at a relatively high rate, but at the same time have a long enough pot life that they can be applied without the need for plural-component impingement mixing equipment. The pot life in general terms is comparable to the drying time of the coating. Once the coating is applied to the substrate, it is exposed to the catalytic influence of ambient water and the curing reaction accelerates. This curing profile permits polyaspartic coatings to be applied manually with a brush or roller. Polyaspartic coating technology should be considered if the application calls for a high-performance coating with a long open time and a fast cure profile.
For additional information on the topics addressed or to ask another question, email jeff.dormish@covestro.com with the subject line “Polyurethane Q&A.”
Any views or opinions expressed in this column are those of the author and do not represent those of ASI, its staff, Editorial Advisory Board or BNP Media.