Applications For Plasma Surface Treatment In The Medical Industry
The selection of a polymer for use in biomedical products such as medical devices, disposables or diagnostic products ultimately depends on the required surface properties of the material in contact with the tissue, fluid or protein. The surface properties required for blood compatibility, for instance, are very different from those required for optimum cell-culturing applications or an adhesive-bonding application. But, the decision to use a particular polymer often has more to do with the economics of one polymer over another and the processing/manufacturing considerations, than the surface property required for optimum product performance.
In other cases, the surface properties may be the most critical criteria and determine the selection of a polymer regardless of its bulk properties. Plasma treatment enables the materials engineer to create the surface properties specific for the application, essentially uncoupling the two often-competing requisites for materials selection: engineering strength and conversion techniques vis-à-vis surface properties.

Gas Plasma
There are many definitions of the term plasma, depending on one’s discipline. It has often been referred to as the fourth state of matter. Its generation is analogous to the transition that occurs when energy is supplied to a solid material, causing it to melt and the liquid to become a gas. Sufficient additional energy supplied to a gas creates a plasma. For the treatment of polymeric material, a “cold gas plasma” is recommended, where the ambient treatment atmosphere is near room temperature (Figure 1). Cold gas plasma is a vacuum process. Typically, plasma is composed of highly excited atomic, molecular, ionic and radical species. Although the electron temperature in plasma can be as high as 5,000°K, because of the vacuum conditions where the number of particles present is very small, the bulk temperature of the gas is essentially ambient.
Plasma Processes
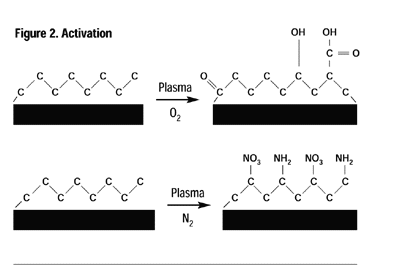
Plasma processes can be conveniently classified into four overall processes: cleaning, activation, grafting and deposition. Cleaning: Inert and oxygen plasmas are ideal for cleaning. The plasma-cleaning process removes, via ablation, organic contaminates such as cutting oils, skin oils and mold releases on the surface of most industrial materials. These surface contaminants undergo repetitive chain scission under the influence of ions, free radicals and electrons of the plasma until their molecular weight is sufficiently low to boil away in the vacuum. To obtain molecular cleanliness, there is not a more effective method than plasma. Activation: When a polymer or elastomer is treated in an inert gas or a non-carbon-containing gas such as oxygen, ammonia or nitrous oxide, to name a few, the primary result is the incorporation of different moieties of the process gas onto the surface of the material being treated. For example, the surface of polyethylene normally consists solely of carbon and hydrogen, however, in an appropriate plasma, the surface may be “activated” to contain a variety of functional groups including, but not limited to, hydroxyl, carbonyl, peroxyl, carboxylic, amino and amines (Figure 2).Almost any polymer or elastomer surface may be modified to provide chemical functionality to specific adhesives or coatings, significantly enhancing the adhesion characteristics and permanency. Polymers activated in such a manner provide greatly enhanced adhesive strength and permanency.

Plasma Surface-Treatment Applications
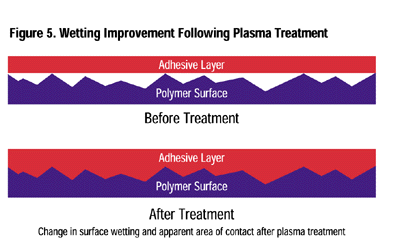
Adhesion Enhancement: Whether it is an adhesive-bonded joint or the permanency of painted or printed decoration and markings, plasma surface treatment can provide significant improvement. The strength of an adhesive joint is enhanced by several factors: removal of contaminants (weak boundary layers), an increase in the surface energy of the substrate (improved wetting), and a chemically reactive site for the adhesive or ink to covalently bond (Figure 5).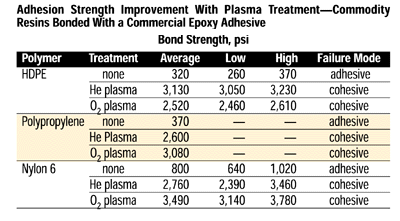
Catheters are routinely used medical devices for surgical and medical care applications. Due to the diversity of applications, (vascular, respiratory, wound care, urethral and renal) catheters are comprised of a variety of components and materials.
Numerous materials have been plasma treated to increase bond strength and improve bond permanency. Plasma treatment can be tailored to effectively work with adhesives typically employed in the medical industry, including but not limited to epoxies, urethanes and cyanate ester resins.
In most instances, the loci of failure shifts with plasma treatment from adhesive-bond failure to cohesive failure, either within the adhesive layer or the substrate being bonded. Adhesion strength improvement is dramatic, as shown below in the table of commodity resins bonded with a commercial epoxy adhesive.
Note that regardless of which plasma was employed, the failure mode shifted from adhesive to cohesive with nearly a 10-fold improvement. Further, the gas process providing the largest increase in strength varied with the substrate; thus, for any combination of substrate and adhesive, maximization of the bond strength requires some process improvement.
Fluoropolymers are typically thought of as a release material to which quality adhesive bonds are impossible. However, with plasma treatment all fluoropolymers can be readily modified to achieve bond strengths in excess of the cohesive strength of the specific fluoropolymer.
Hydrogen plasma, often in conjunction with a co-process gas, has been found to be particularly effective. Plasma processes employing hydrogen cause dehydrohalogenation along the fluoropolymer backbone to which the co-process gas can subsequently covalently attach.4
A commercial product line of fluoropolymers with a variety of pressure sensitive adhesives, called Fluorogrip5, has recently been introduced to the marketplace. These fluoropolymer films, when applied to the interior of tanks, splash zones and feed hoppers of aggressive environments, have been shown to provide excellent protection and little loss of adhesion when immersed in a wide range of aggressive chemicals and solvents.
Surface Energy
Depending on the gas employed, plasma treatment may render the surface very hydrophilic, oleophobic or hydrophobic. The hydrophilicity can be controlled often within a few dyne-cm. Oleo- and hydrophobicity are readily achieved in plasmas containing fluorine. If a fluoro-alkane such as tetrafluoromethane or hexafluoroethane is utilized as the process gas, fluorine will be substituted for abstracted hydrogen on the surface of the substrate, reducing its surface energy.
Reactive Handles
The specific chemical functionality can be controlled depending on the choice of co-reactant process gases.6 In one series, the chemical functionality was compared using plasmas of CO2/CH3OH (carbon dioxide/ methanol), O2/CH3COCH3 (oxygen/acetone) and CO2/N2O (carbon dioxide/nitrous oxide). The total oxygen content as determined by ESCA was similar in all cases, however the relative concentration of hydroxyl, carboxyl and carbonyl was significantly different.Such surface “functionalization” may aid in the attachment of heparin to normally hydrophobic polymer or elastomer surfaces.7,8 Functionaliza-tion may also be very important for subsequent, traditional wet-chemical modification of the surface, such as hydrolyzing silane-coupling agents and transesterification of acid-terminated coatings.
Plasma-induced grafting offers another method of providing specific reactive sites to normally inert polymers. When an unsaturated monomer such as allyl alcohol or allyl amine is introduced into the reaction chamber after the plasma is extinguished but prior to venting to atmosphere, it will add to the free radical, yielding a grafted polymer.
One advantage over activation is that the concentration of the desired functionality is better controlled, i.e., allyl alcohol primarily provides hydroxyl functionality, acrylic acid primarily provides carboxylic functionality and allyl amine primarily provides amine functionality. A variety of porous membranes have been functionalized in this manner to allow for subsequent adhesion (binding) of DNA.9,10
Summary
Even though plasma is a vacuum process, thus considered a batch system, there are many automated systems in use coupled to continuous production lines. Plasma systems of all sizes and degrees of automation are available, from manually loaded batch systems as small as a dormitory-room microwave oven to fully automated systems treating automotive instrument panels and fascia.Cold gas plasma processes offer an efficient and reliable means to alter surface properties of all materials without affecting the bulk properties of the treated material. Reengineering the polymer surface by introducing adhesive-reactive functional groups in a controlled and reproducible manner greatly enhances adhesive performance, permanency and reliability.
The nature of cold gas plasma surface modification lends itself to precise control and process repeatability. In the vast majority of applications, plasma surface treatment employs innocuous gases, enabling the manufacturing engineer to avoid corrosive chemicals and solvents. Plasma surface treatment is recognized by the State of California as a clean air technology. It is a workplace and environmentally friendly technology. Shouldn’t you consider what plasma treatment may do for you?
Additional information on plasma treatment systems is available from 4th State, Inc., 1260 Elmer St., Belmont, CA 94002-2806; call 650-596-1600; fax 650-596-1604; visit the Web site www.4thstate.com; or email cynthia@4thstate.com
Links
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!


