Stretching Polychloroprene Supplies in Solventborne Contact Cements
Phenolic resins have been a crucial part of solventborne polychloroprene adhesives for more than 50 years, and provide performance enhancements that allow these adhesives to be used in very demanding applications. They are made by reacting a phenolic resin with magnesium oxide to form a metal resinate, and they are commonly referred to as “contact cements.”

Phenolic resins have been a critical part of solvent borne polychloroprene adhesives for more than 50 years, and they provide performance enhancements allowing these adhesives to be used in very demanding applications. The phenolic resin is typically reacted with magnesium oxide to form a metal resinate. This results in adhesives exhibiting high temperature performance with improved adhesive and cohesive strength. The metal resinate also allows polychloroprene adhesives to bond with itself instantly and permanently when surfaces coated with adhesive are brought together. Adhesives of this type are commonly referred to as “contact cements.” Contact cements can be prepared across a broad range of solids and viscosities, and can be applied by spraying, rolling, or brushing on to the substrates being adhered. Similar products at higher solids can be extruded and used as caulks and sealants. Other resins can be used in solvent based polychloroprene adhesives to increase the open time, the working time between when the adhesive is applied and when the substrates are brought together. Some customers have also tried using inexpensive materials like hydrocarbon resins and rosin to decrease costs, but this typically reduces the performance with regards to bond strength and high temperature performance.
The most common phenolic resin used to make solvent borne polychloroprene adhesives are those made by reacting para-tertiary butylphenol (PTBP) with formaldehyde under basic conditions. The base is neutralized and washed out of the resin, and the resulting mixture is distilled and polymerized to form a solid resinous product that has reactive groups called methylol groups (a.k.a hydroxymethyl groups). These functional groups allow the resin to complex with magnesium oxide to form magnesium resinate. Alternate alkylated phenols such as those made from para-tertiary octylphenol can be using by the same method to prepare resins with different solubility characteristics. It is also possible to alter the physical structure of the resin by controlling the processing conditions. Thus, resins made from the same raw materials can have different levels of activity and different molecular weights. SI Group, Inc. manufactures many different resins based on PTBP, and several commercial resins were compared both alone and in combination with other resins in this study.
It is also possible to use different reaction conditions to prepare resinous materials from alkylated phenols that do not have any reaction methylol groups. This type of resin, commonly called a novolak resin, does not react with magnesium oxide. Alkylphenol novolaks are commonly used to tackifier rubber for making in tires and rubber goods, but have also been used in various types of adhesives to extend open time. Different alkylated phenols are typically used to control the relative solubility of the resin in the rubber, and SI Group, Inc. makes many different grades. Several different commercial products were used in combination with reactive resins in this study.
A third type of phenolic resin can be made by reacting phenol with terpenes, materials that are derived from natural sources such as pine trees. This class of resin is typically referred to as a terpene phenolic resin. Terpene phenolic resins are most frequently used in hot melt adhesives to increase tack. They are of very low molecular weight and have high softening points. They also have the advantage of being made from renewable materials and do not contain formaldehyde. Terpene phenolic resins are frequently cited for use as additives in solvent borne adhesives to increase the open time without decreasing high temperature performance.

The rubber base is mixed overnight to insure complete dissolution of the rubber. The pre-reaction is carried out separately in an Erlenmeyer flask to insure complete reaction of the resin with the magnesium oxide. The two solutions are blended to make the finished adhesive.
The first test procedure used to screen various resin combinations was dynamic peel under at two different temperatures. For this test, two one-inch strips of canvas were coated with adhesive for most of there length and put together using a standard pressure. When assembled, the uncoated portions of the canvas were folded away from the bonded portion in opposite directions, forming a “T” shaped assembly. After aging, these specimens were attached at the two tabs of the “T” and pulled apart by an Instron® tensile tester by force that is perpendicular to the adhesive bond. This test is commonly referred to as a “T-Peel” test. The use of two temperatures allows comparison of performance at ambient temperature and the normal high end for objects used in an environment occupied by people, such as automotive interiors.
The second test procedure used is designed to determine how long the coated substrates can be kept apart before final assembly occurs. Typically, the bond strength will rise to a maximum as the solvent evaporates away and then decline after a period of time. For this test, a steel panel is used as one substrate, and one-inch strips of canvas are used as the second substrate. Four strips of canvas are coated simultaneously, and applied sequentially to the steel panel after different periods of time. In this case, the time periods were 30, 60, 90, and 120 minutes. The assembly is aged and heated to 121°C (250°F). The strips are pulled in a direction parallel to the adhesive bond using an Instron® tensile tester, and the relative force is recorded. This is commonly called a high temperature shear test.
The third test used to test adhesives is designed to evaluate how the adhesive will perform at very high temperatures. Test specimens are prepared in the same manner as the dynamic peel test, but are marked in ½ centimeter increments the length of the adhesive bond. One tab of the assembly is attached to the top of an oven, and the other end is left hanging. The hanging end has a 1250 gram weight attached to it, and the oven door is then closed. The oven is heated at a constant rate of 2°C per minute, and the bond is observed. As the temperature rises, the bond starts to gradually peel apart until the total bonded area has been separated. A graph is generated with the temperature on the vertical axis and the level of separation on the horizontal axis. This test is somewhat limited by the temperature that the canvas substrate starts to char.

For this study, different reactive resins were used alone and in combination with other resins to determine the relative performance. (See Table 2.)

Standard load: 45 parts resin, 4.5 parts MgO
Slight Increase: 50 parts resin, 5.0 parts MgO
Moderate increase: 75 parts resin, 7.5 parts MgO
Modified Moderate: 75 parts resin, 5.0 parts MgO
 Higher resin content by itself gives shorter open time (90 or 60 minutes), but when combined with a smaller percentage of MgO per resin content, open times of at least 2 hours are obtained (equal to the blank).
Higher resin content by itself gives shorter open time (90 or 60 minutes), but when combined with a smaller percentage of MgO per resin content, open times of at least 2 hours are obtained (equal to the blank).
 All adhesives made with higher resin content gave improved high temperature properties. Note that the failure temperature is where the canvas starts to char (200°C).
All adhesives made with higher resin content gave improved high temperature properties. Note that the failure temperature is where the canvas starts to char (200°C).
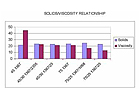
 It can be noted that the viscosity drops when more resin is used.
It can be noted that the viscosity drops when more resin is used.

Rezilite® 888 at 45 parts, SP-1068 at 30 parts
Rezilite® 888 at 65 parts, SP-1068 at 10 parts
Rezilite® 888 at 45 parts, SP-553 at 25 parts
Rezilite® 888 at 45 parts, SP-560 at 25 parts
 The longest open time of at least 2 hours is obtained for SP-560 combined with Rezilite® 888. Using less PTOP tackifier SP-1068 with higher levels of Rezilite® 888 gives an optimal open time of 90 minutes.
The longest open time of at least 2 hours is obtained for SP-560 combined with Rezilite® 888. Using less PTOP tackifier SP-1068 with higher levels of Rezilite® 888 gives an optimal open time of 90 minutes.
 Normal levels of Rezilite® 888 with high levels of PTOP tackifier resin give dramatic decline in failure temperature. However, using more Rezilite® 888 and low levels of PTOP tackifier resin gives better high temperature performance than the standard. Modification with terpene phenolic resins does not adversely affect high temperature performance by very much, but allow for a significant increase in quantity of adhesive made with a given quantity of polychloroprene.
Normal levels of Rezilite® 888 with high levels of PTOP tackifier resin give dramatic decline in failure temperature. However, using more Rezilite® 888 and low levels of PTOP tackifier resin gives better high temperature performance than the standard. Modification with terpene phenolic resins does not adversely affect high temperature performance by very much, but allow for a significant increase in quantity of adhesive made with a given quantity of polychloroprene.
 General trend is for higher solids to equal higher viscosity. SP-553 showed drop in viscosity versus SP-560.
General trend is for higher solids to equal higher viscosity. SP-553 showed drop in viscosity versus SP-560.

HRJ-1367 at 45 parts (BLANK)
HRJ-1367 at 45 parts, HRJ-2355 at 30 parts
HRJ-1367 at 45 parts, SP-25 at 30 parts
HRJ-1367 at 75 parts
HRJ-1367 at 75 parts, SP-1068 at 25 parts
HRJ-1367 at 75 parts, SP-25 at 25 parts
 For increased resin loads, high levels of HRJ-1367 by itself were the best alternative for getting a long open time. SP-25 showed better performance than HRJ-2355 at a low load of HRJ-1367. SP-25 did not give as long open times compared to SP-1068 when combined with HRJ-1367 at higher loads. However, the shear adhesion across the entire period of time did not show significant decline.
For increased resin loads, high levels of HRJ-1367 by itself were the best alternative for getting a long open time. SP-25 showed better performance than HRJ-2355 at a low load of HRJ-1367. SP-25 did not give as long open times compared to SP-1068 when combined with HRJ-1367 at higher loads. However, the shear adhesion across the entire period of time did not show significant decline.
 Every combination at high levels of resin performed better than the standard load. In this study, the best performance was obtained by using 75 parts of HRJ-1367 with 25 parts of SP-25. This was slightly better than using 75 parts of HRJ-1367 by itself. SP-25 also showed better performance than HRJ-2355, indicating that the mixed alkylphenol tackifier resin is the best choice for improving polychloroprene adhesives.
Every combination at high levels of resin performed better than the standard load. In this study, the best performance was obtained by using 75 parts of HRJ-1367 with 25 parts of SP-25. This was slightly better than using 75 parts of HRJ-1367 by itself. SP-25 also showed better performance than HRJ-2355, indicating that the mixed alkylphenol tackifier resin is the best choice for improving polychloroprene adhesives.
 Higher resin content results in lower viscosity, even at higher solids.
Higher resin content results in lower viscosity, even at higher solids.

SP-103
HRJ-1367
Rezilite® 888
Blank (Rezilite® 888 at 45 parts)
All of these resins are commonly sold into the solvent based polychloroprene contact cement market, and are well known for providing particular levels of performance at the normal level of 45 parts of resin and 4.5 parts of magnesium oxide. In this case, the same pre-reaction was formed (10 parts MgO per 100 parts of resin), but a larger quantity of pre-reaction was added to the rubber base.
 An open time of 2 hours (120 minutes) was obtained for HRJ-1367 at high resin content. Surprisingly, the lower molecular weight resin SP-103 did not have as long an optimal open time.
An open time of 2 hours (120 minutes) was obtained for HRJ-1367 at high resin content. Surprisingly, the lower molecular weight resin SP-103 did not have as long an optimal open time.
 It is quite notable that Rezilite® 888 gave extremely good results in this test. HRJ-1367 also performed much better than the blank at 45 parts of resin. As expected, low molecular weight SP-103 did not perform any better than the blank.
It is quite notable that Rezilite® 888 gave extremely good results in this test. HRJ-1367 also performed much better than the blank at 45 parts of resin. As expected, low molecular weight SP-103 did not perform any better than the blank.
 Higher resin content decreases viscosity, even at higher solids. HRJ-1367 gives higher viscosity at both low and high resin content compared to Rezilite® 888.
Higher resin content decreases viscosity, even at higher solids. HRJ-1367 gives higher viscosity at both low and high resin content compared to Rezilite® 888.

Rezilite® 888 with SP-1045
Rezilite® 888 with HRJ-1367
HRJ-1367 with SP-103
HRJ-1367 with SP-1045
These blends were compared to normal loads of Rezilite® 888 and HRJ-1367.
 The best open time came from the combination of SP-103 and HRJ-1367. This indicates that the ideal blend for a long open time comes from a mixture of high and low reactivity PTBP resins at a low molecular weight.
The best open time came from the combination of SP-103 and HRJ-1367. This indicates that the ideal blend for a long open time comes from a mixture of high and low reactivity PTBP resins at a low molecular weight.
 The best performance comes from the combination of Rezilite® 888 and HRJ-1367. In general, using HRJ-1367 in combination with any other heat reactive resin gave very good results.
The best performance comes from the combination of Rezilite® 888 and HRJ-1367. In general, using HRJ-1367 in combination with any other heat reactive resin gave very good results.
 Using higher resin loads significantly reduces the viscosity of the finished adhesive.
Using higher resin loads significantly reduces the viscosity of the finished adhesive.
Specific resin combinations can be used to improve performance above the normal adhesives being produced today.
In the event that extremely high heat resistance is needed, the combination of Rezilite® 888 and HRJ-1367 at resin loads of 50 parts each per 100 parts of rubber gave the best performance. The best result was obtained when 5 parts of MgO was pre-reacted with 100 parts of total resin.
Open time is generally decreased by increasing the resin load. However, careful choice of tackifier resins can bring the open time back to normal levels.
The best choice for improving performance from among the alkylphenol tackifier resins was SP-25. This resin has not been traditionally used in polychloroprene adhesives, but it was found to give good results in this study.
Use of increased levels of phenolic resin shifts the viscosity downward. This can allow the adhesive maker to make higher solids adhesives that are dispensed from canisters, thus reducing VOC’s. In the event that the viscosity cannot be altered, some type of thickening agent will have to be used. Use of thickening agents was beyond the scope of this study.

In markets such as leather goods, it is critical to have strong and quick bonds with short open times.
Introduction
Polychloroprene rubber (PCR) grades used in solvent borne adhesives have become in short supply due to a combination of demand and plant closings. Additional plant closings have been announced, and some grades of PCR will no longer be available in the near future. At the same time, demand for polychloroprene rubber based adhesives remains strong. While there is investment in new capacity of PCR, it is targeted for grades that are not used in solvent borne adhesives. This is resulting in a market dynamic where most adhesive formulators are seeing higher prices to get enough PCR, some cannot get enough polychloroprene rubber at any price, and others struggle to get any PCR at all. Different regions are served by different suppliers of PCR, and existing formulators are getting preference over new users. However, even long term formulators cannot get more PCR than they purchased in the past to avoid opportunistic buying and re-selling. Thus, formulators who develop new business are often in the position of not being able to get enough of critical raw materials.Phenolic resins have been a critical part of solvent borne polychloroprene adhesives for more than 50 years, and they provide performance enhancements allowing these adhesives to be used in very demanding applications. The phenolic resin is typically reacted with magnesium oxide to form a metal resinate. This results in adhesives exhibiting high temperature performance with improved adhesive and cohesive strength. The metal resinate also allows polychloroprene adhesives to bond with itself instantly and permanently when surfaces coated with adhesive are brought together. Adhesives of this type are commonly referred to as “contact cements.” Contact cements can be prepared across a broad range of solids and viscosities, and can be applied by spraying, rolling, or brushing on to the substrates being adhered. Similar products at higher solids can be extruded and used as caulks and sealants. Other resins can be used in solvent based polychloroprene adhesives to increase the open time, the working time between when the adhesive is applied and when the substrates are brought together. Some customers have also tried using inexpensive materials like hydrocarbon resins and rosin to decrease costs, but this typically reduces the performance with regards to bond strength and high temperature performance.
Premise for the Study
It was proposed that increasing the quantity of select phenolic resins could extend the quantity of adhesive made with a given quantity of polychloroprene rubber. To do this, increased loads of the resin already being used were added to the adhesive. In addition, combinations of phenolic resins with different chemistries were used to make adhesives. Combined levels of phenolic resins were used to produce adhesives at 75 and 100 parts of resin per 100 parts of PCR. Since the rubber and the resin are the main ingredients that contribute to the solids content of the adhesive, increasing the resin load to 75 parts or resin per 100 parts of PCR results in about one fifth more adhesive at the same solids content. By increasing the resin load to 100 parts of resin per 100 parts of PCR, about one third more adhesive can be made at the same solids content.Background on Use of Phenolic Resins in PCR Adhesives
Solvent borne polychloroprene rubber adhesives are typically made in two parts. The first part is a solution of polychloroprene rubber, metal oxides, and antioxidant dissolved into solvent at 16-17 percent solids content. The low solids content is necessary due to the high viscosity of the rubber solution. The second part is a solution of resin that has been reacted with magnesium oxide, and this is typically prepared at 50% solids. The two solutions are combined with mixing to prepare the finished adhesive. Alternatively, some customers use churns which are capable of mixing all of the ingredients together at the same time. It is well documented that the highest performance adhesives are prepared using the 2-part system, so that is the technique that was used in this study.The most common phenolic resin used to make solvent borne polychloroprene adhesives are those made by reacting para-tertiary butylphenol (PTBP) with formaldehyde under basic conditions. The base is neutralized and washed out of the resin, and the resulting mixture is distilled and polymerized to form a solid resinous product that has reactive groups called methylol groups (a.k.a hydroxymethyl groups). These functional groups allow the resin to complex with magnesium oxide to form magnesium resinate. Alternate alkylated phenols such as those made from para-tertiary octylphenol can be using by the same method to prepare resins with different solubility characteristics. It is also possible to alter the physical structure of the resin by controlling the processing conditions. Thus, resins made from the same raw materials can have different levels of activity and different molecular weights. SI Group, Inc. manufactures many different resins based on PTBP, and several commercial resins were compared both alone and in combination with other resins in this study.
It is also possible to use different reaction conditions to prepare resinous materials from alkylated phenols that do not have any reaction methylol groups. This type of resin, commonly called a novolak resin, does not react with magnesium oxide. Alkylphenol novolaks are commonly used to tackifier rubber for making in tires and rubber goods, but have also been used in various types of adhesives to extend open time. Different alkylated phenols are typically used to control the relative solubility of the resin in the rubber, and SI Group, Inc. makes many different grades. Several different commercial products were used in combination with reactive resins in this study.
A third type of phenolic resin can be made by reacting phenol with terpenes, materials that are derived from natural sources such as pine trees. This class of resin is typically referred to as a terpene phenolic resin. Terpene phenolic resins are most frequently used in hot melt adhesives to increase tack. They are of very low molecular weight and have high softening points. They also have the advantage of being made from renewable materials and do not contain formaldehyde. Terpene phenolic resins are frequently cited for use as additives in solvent borne adhesives to increase the open time without decreasing high temperature performance.

Standard Adhesive Formulation
Polychloroprene adhesives can be made from many different grades of polychloroprene. For this study, Neoprene® AD 20 made by DuPont Performance Elastomers was used. This is a mid-range viscosity grade of polychloroprene noted for fast crystallizing properties and similar grades are offered by other global PCR manufacturers. The solvent system was a blend of toluene, methyl ethyl ketone, and hexane at equal weights. Elastomag® 170S, a highly reactive grade of magnesium oxide supplied by Rohm & Haas, was used in order to provide uniform results. The elastomer was milled with magnesium oxide, zinc oxide, and a phenolic anti-oxidant prior to dissolving in the solvent system. The phenolic resin was pre-reacted with magnesium oxide in the same solvent blend. Normal loads for the blank are shown in Table 1.The rubber base is mixed overnight to insure complete dissolution of the rubber. The pre-reaction is carried out separately in an Erlenmeyer flask to insure complete reaction of the resin with the magnesium oxide. The two solutions are blended to make the finished adhesive.
Physical Property Testing
The viscosity of the finished adhesive is also a very important property. In some applications, such as with spray grades of adhesive, it is essential to have a low viscosity. Other types of adhesives, such as those applied to vertical surfaces, must be viscous enough to stay in place. The solids content is directly related to viscosity and is also an important property, since solids content is closely related to cost. All of the adhesives in this study were tested for both viscosity and solids content. The test results for solids content are reported in percent, and the results for viscosity are reported in centipoises. In order to get the two properties on the same graph, the viscosity in centipoises was divided by 40.Performance Testing Protocols
Testing procedures for adhesives made from polychloroprene are designed to look at different properties that are important to the end-user. In some markets, such as post-formed countertops, bond retention at high temperatures is a critical requirement. In other markets, such as making shoes and leather goods, it is most critical to have strong and quick bonds with short open times. When making adhesives for large assemblies, such as single ply rubber roofing, it is critical to have long open times and very aggressive adhesion under severe conditions for very long periods of time.The first test procedure used to screen various resin combinations was dynamic peel under at two different temperatures. For this test, two one-inch strips of canvas were coated with adhesive for most of there length and put together using a standard pressure. When assembled, the uncoated portions of the canvas were folded away from the bonded portion in opposite directions, forming a “T” shaped assembly. After aging, these specimens were attached at the two tabs of the “T” and pulled apart by an Instron® tensile tester by force that is perpendicular to the adhesive bond. This test is commonly referred to as a “T-Peel” test. The use of two temperatures allows comparison of performance at ambient temperature and the normal high end for objects used in an environment occupied by people, such as automotive interiors.
The second test procedure used is designed to determine how long the coated substrates can be kept apart before final assembly occurs. Typically, the bond strength will rise to a maximum as the solvent evaporates away and then decline after a period of time. For this test, a steel panel is used as one substrate, and one-inch strips of canvas are used as the second substrate. Four strips of canvas are coated simultaneously, and applied sequentially to the steel panel after different periods of time. In this case, the time periods were 30, 60, 90, and 120 minutes. The assembly is aged and heated to 121°C (250°F). The strips are pulled in a direction parallel to the adhesive bond using an Instron® tensile tester, and the relative force is recorded. This is commonly called a high temperature shear test.
The third test used to test adhesives is designed to evaluate how the adhesive will perform at very high temperatures. Test specimens are prepared in the same manner as the dynamic peel test, but are marked in ½ centimeter increments the length of the adhesive bond. One tab of the assembly is attached to the top of an oven, and the other end is left hanging. The hanging end has a 1250 gram weight attached to it, and the oven door is then closed. The oven is heated at a constant rate of 2°C per minute, and the bond is observed. As the temperature rises, the bond starts to gradually peel apart until the total bonded area has been separated. A graph is generated with the temperature on the vertical axis and the level of separation on the horizontal axis. This test is somewhat limited by the temperature that the canvas substrate starts to char.

Description of Resins Used
All of the resins used in this study are commercial products produced by SI Group, Inc. or affiliated companies.For this study, different reactive resins were used alone and in combination with other resins to determine the relative performance. (See Table 2.)

Chart 1. Dynamic Peel. Increased levels of Rezilite® 888 gives higher room temperature and elevated temperature performance. When combined with reduced percentage of MgO gave best overall results.
Trial 1: Different Loads of a Single Resin (Rezilite 888)
Comparison of adhesives made with different levels of a single heat reactive resin. Rezilite® 888 was used at different levels of resin and different levels of magnesium oxide.Standard load: 45 parts resin, 4.5 parts MgO
Slight Increase: 50 parts resin, 5.0 parts MgO
Moderate increase: 75 parts resin, 7.5 parts MgO
Modified Moderate: 75 parts resin, 5.0 parts MgO

Chart 2. Shear Adhesion/Open Time Comparison.

Chart 3. High Temperature Performance.

Chart 4. Solids/Viscosity Relationship.

Chart 5. Dynamic Peel. High levels of PTOP tackifier diminish bond strength, but increasing Rezilite® 888 and using low levels of tackifier gives better high temperature performance. Both SP-553 and SP-560 give improved high temperature results. SP-553 gives better room temperature results as well.
Trial 2: Combination of Reactive Resin with Tackifier Resins
Rezilite® 888 at 45 parts, no additional resins (BLANK)Rezilite® 888 at 45 parts, SP-1068 at 30 parts
Rezilite® 888 at 65 parts, SP-1068 at 10 parts
Rezilite® 888 at 45 parts, SP-553 at 25 parts
Rezilite® 888 at 45 parts, SP-560 at 25 parts

Chart 6. Shear Adhesion/Open Time Comparison.

Chart 7. High Temperature Performance.

Chart 8. Solids/Viscosity Relationship.

Table 9. In all cases, adding more resin increased the dynamic peel at elevated temperatures over the standard load of 45 parts of resin. SP-25 also increased peel strength at room temperature when compared to the same quantity of HRJ-1367 without tackifier resin. Based on this data, SP-25 (mixed alkylphenol resin) is the best candidate for maintaining or increasing dynamic peel.
Trial 3: Differentiation of Alkyphenolic Tackifier Resins
Three different alkylphenolic tackifier resins were used with another heat reactive resin, HRJ-1367, in the following combinations to determine which would provide the best properties:HRJ-1367 at 45 parts (BLANK)
HRJ-1367 at 45 parts, HRJ-2355 at 30 parts
HRJ-1367 at 45 parts, SP-25 at 30 parts
HRJ-1367 at 75 parts
HRJ-1367 at 75 parts, SP-1068 at 25 parts
HRJ-1367 at 75 parts, SP-25 at 25 parts

Chart 10. Shear Adhesion/Open Time Comparison.

Chart 11. High Temperature Performance.

Chart 12. Solids/Viscosity Relationship.

Chart 13. Dynamic Peel. Dynamic peel results are much improved by using more resin, both at room temperature and elevated temperature. The best results were obtained by using HRJ-1367 at 75 parts.
Trial 4: Comparison of Different Single Resins at High Loads
SI Group offers a number of different resins made from PTBP to the adhesive market. In this study, a wide range of resins was tested at the 75 part level, which allows the compounder to make about 20% more adhesive from a given supply of polychloroprene. The resins tested are the following:SP-103
HRJ-1367
Rezilite® 888
Blank (Rezilite® 888 at 45 parts)
All of these resins are commonly sold into the solvent based polychloroprene contact cement market, and are well known for providing particular levels of performance at the normal level of 45 parts of resin and 4.5 parts of magnesium oxide. In this case, the same pre-reaction was formed (10 parts MgO per 100 parts of resin), but a larger quantity of pre-reaction was added to the rubber base.

Chart 14. Shear Adhesion/Open Time Comparison.

Chart 15. High Temperature Performance

Chart 16. Solids/Viscosity Relationship.

Chart 17. Dynamic Peel. Using any of the combinations of resin with total resin load of 100 parts gave better high temperature performance than the standard products at normal levels. The combination of Rezilite® 888 and HRJ-1367 gave particularly good results, with very little decline in bond strength at the higher temperature.
Trial 5: Mixtures of Heat Reactive Resins
The following combinations of heat reactive resins were used to make adhesives with 100 parts of total resin per 100 parts of rubber. This provides more than 35% more adhesive from the same quantity of polychloroprene when only 45 parts of resin is used. In this study, solutions of two resins were made at 50 parts each, and 5 parts of MgO was co- reacted with the two resins together. Due to the overall reduction in the percentage of MgO per part of resin, the adhesives formed were clear instead of opaque. Combinations tested were the following:Rezilite® 888 with SP-1045
Rezilite® 888 with HRJ-1367
HRJ-1367 with SP-103
HRJ-1367 with SP-1045
These blends were compared to normal loads of Rezilite® 888 and HRJ-1367.

Chart 18. Shear Adhesion/Open Time Comparison.

Chart 19. High Temperature Performance.

Chart 20. Solids/Viscosity Relationship.
Conclusions
The premise that performance properties could be retained for contact cements was proven for individual resins at nearly double the normal load, and for resin combinations at more than double the normal load. Thus, it is indeed possible to increase the quantity of adhesive made from a fixed quantity of polychloroprene rubber without sacrificing performance.Specific resin combinations can be used to improve performance above the normal adhesives being produced today.
In the event that extremely high heat resistance is needed, the combination of Rezilite® 888 and HRJ-1367 at resin loads of 50 parts each per 100 parts of rubber gave the best performance. The best result was obtained when 5 parts of MgO was pre-reacted with 100 parts of total resin.
Open time is generally decreased by increasing the resin load. However, careful choice of tackifier resins can bring the open time back to normal levels.
The best choice for improving performance from among the alkylphenol tackifier resins was SP-25. This resin has not been traditionally used in polychloroprene adhesives, but it was found to give good results in this study.
Use of increased levels of phenolic resin shifts the viscosity downward. This can allow the adhesive maker to make higher solids adhesives that are dispensed from canisters, thus reducing VOC’s. In the event that the viscosity cannot be altered, some type of thickening agent will have to be used. Use of thickening agents was beyond the scope of this study.
Planned Additional Work
Additional work is planned to extend the database used for this report. This will include more resins made by SI Group around the world. Higher levels of single resins will also be included. If the reader has any suggestions of work that should be considered, please contact the author at james.lamb@siigroup.com and give as much detail as possible.Links
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!


