HOT-MELT FILMS
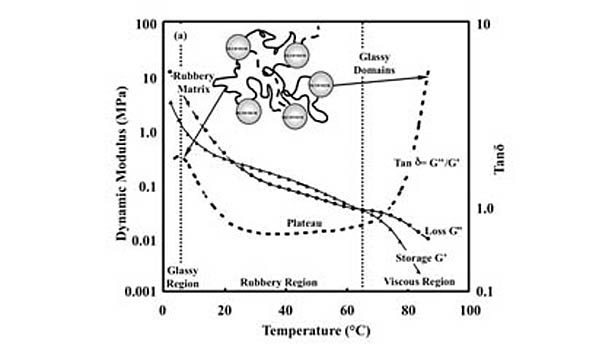
The term hot melt simply refers to the ability of the adhesive to melt upon heating, allowing it to be spread or coated onto a substrate. In practice, this terminology typically refers to PSAs that employ block copolymers, which combine styrene segments with those composed of monomers possessing lower glass-transition temperatures in their homopolymer form. These so-called rubbery blocks are often composed of ethylene-propylene, ethylene-butene, isoprene or butadiene. The incompatibility of the styrenic and rubbery blocks provides these polymers with separate microphases and phase transitions, which are apparent in dynamic mechanical analysis (DMA) thermoscans.
Figure 1 shows DMA data for a commercial hot-melt PSA measured over a broad temperature range (X = 10 rad/s, shear mode). The lower temperature transition is the glass transition for the rubbery blocks; the higher temperature transition is associated with the styrene phase glass transition and/or disruption temperature at which the interactions between styrene functional groups that provide the residual cohesive strength (sometimes described as physical crosslinks) are mostly eliminated.
Figure 1 also shows the location of the shear adhesion failure temperature (SAFT). The SAFT can be described as the temperature at which highly rapid deformation is found for the PSA, producing a failure response. In practice, it is an indication of the temperature limit on the PSA for adhesive applications. SAFT is commonly measured by laminating identical PSA labels (films plus carriers) to produce an overlap region of 1 in.2.13-14 A constant load is applied to induce a shear stress parallel to the overlapping films, and the temperature is increased until the laminate fails. This temperature is reported as the SAFT.
PSA performance requires a balance between adhesive and cohesive strength. In other words, the adhesive must be soft enough to flow into a surface at low pressures to wet it, but strong enough to withstand various loads without failing. For a hot-melt PSA, this balance is achieved within the plateau region of the adhesive (see Figure 1), which is bracketed by its phase transitions.10 The cohesive strength of a hot-melt PSA declines with increasing temperature throughout the plateau region.
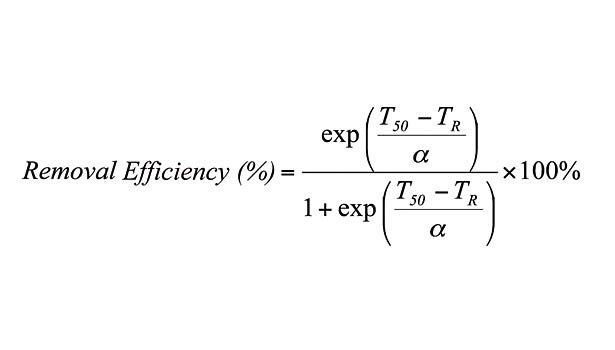
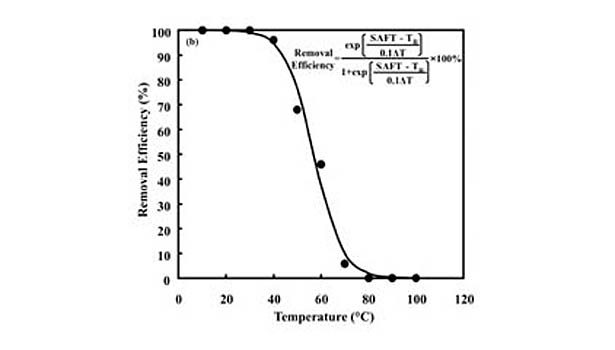
This loss of strength corresponds to an increase in the degree of fragmentation of PSA films during repulping operations, as well as a decrease in their removal via screening operations. In fact, removal efficiency drops from 100% to nearly 0% over the plateau region.10 This behavior is fit by an empirical sigmoidal function relating screening removal efficiency to repulping temperature (TR) of the form (see equation), for which α determines the width of the sigmoidal curve and T50 is its inflection point corresponding to a removal efficiency of 50%. In addition, linear correlations were found between and the thermal width of the plateau region (ΔT) and between T50 and SAFT. Thus, an empirical predictive equation is formed by substituting these parameters into the equation.
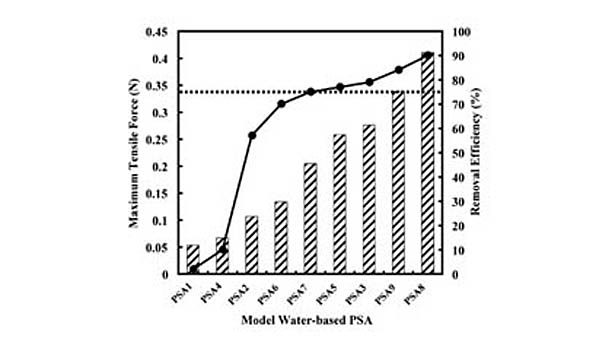
The effectiveness of this approach for predicting screening removal efficiencies was shown previously with both model and commercial hot-melt PSAs developed with various types and concentrations of tackifying resins, processing oils, and styrenic block copolymers.10 An example of the data collected in this study can be seen in Figure 2, which shows the fit of laboratory repulping and screening data for a commercial hot-melt PSA. The data are fit by the model using the thermal width of the plateau region and the SAFT for the PSA.
The general applicability of this predictive equation simply demonstrates the importance of the styrene transition corresponding to a loss in its strength contribution as measured by quantities such as SAFT. Specifically, modifications that increase the SAFT relative to temperatures at which the PSA is being repulped, which typically occurs between 45 and 50°C, will reduce the extent to which it is fragmented and increase its screening removal efficiency.
WATER-BASED FILMS
Water-based adhesives are formulated and processed as aqueous dispersions. The adhesive polymer is produced via emulsion polymerization, which requires the emulsification of reacting monomers and generates a latex dispersion. This colloid or neat latex can serve as the basis for numerous PSAs produced through the addition of different types and concentrations of tackifying dispersions and additives (e.g., wetting agents, defoamers and rheology modifiers) that facilitate coating operations.
Δ
Δ