Options for Polyurethane Chemistry
The use of aliphatic diisocyanates and their derivatives can provide many benefits for thermoplastic materials.



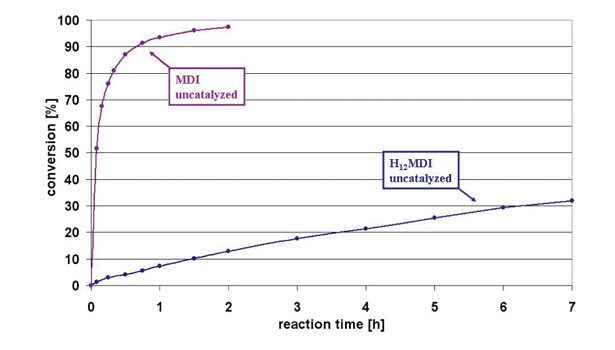
Figure 2. Reaction Profiles of Catalyzed and Uncatalyzed H12MDI Against MDI with n-Octanol at 80°C
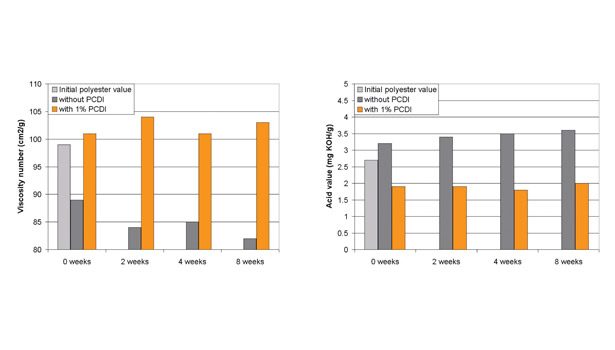
Figure 3. Viscosity Numbers and Acid Values of PCDI-Stabilized Polyesters Stored at 50°C Under Dry Conditions

Figure 4. Viscosity Numbers and Acid Values of PCDI-Stabilized Polyesters Stored at 50°C and 90% Humidity
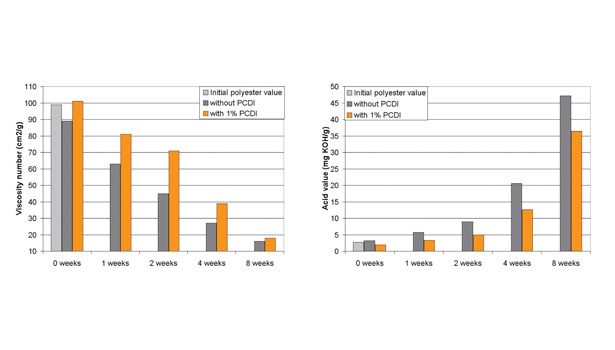
Figure 5. Viscosity Numbers and Acid Values of PCDI-Stabilized Polyesters Stored at 70°C and 90% Humidity










As a material class, polyurethanes have unique properties over many other polymeric materials. Ease of reaction is one inherent property of both aromatic and aliphatic diisocyanates (ADIs), as they react in a 1:1 NCO:OH ratio with the numerous types of polyols available in the commercial market. The formed polyurethane bonds have high chemical resistances against many environmental conditions. Depending on the reactive polyol and the structure of the diisocyanate, polyurethanes can provide excellent abrasion resistance, superb elasticity, and high hardness.
Polyurethane chemistry provides many options in choosing systems that give soft-feel coatings and tough elastomers and rigid foams. But choosing the right systems requires a fundamental understanding of the makeup of the diisocyanates used in the crosslinking. Recent data explore the substitution of an aromatic diisocyanate by an aliphatic diisocyanate, with particular emphasis on the formulation of thermoplastic polyurethanes (TPU) and some additional information on the performance of polycarbodiimides (PCDI).
ALIPHATIC VS. AROMATIC DIISOCYANATES
The use of aromatic vs. aliphatic diisocyanates differs dramatically; that difference has not changed much over the previous decades. It is estimated that only 3% of all diisocyanates sold this year will be aliphatic.1 While ADIs are used in adhesives and sealants, their principal use is in coatings (chiefly aromatics) and—to a small extent—elastomers. Some elastomer uses could benefit from increased outdoor durability. But how should ADIs be formulated to replace aromatic diisocyanates and retain the desired elastomeric properties?
The principal reasons behind this niche position are cost and outdoor durability. While methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) are much lower in cost compared to ADIs, polyurethanes based on MDI and TDI will begin to yellow and then degrade if exposed to UV radiation for any significant period of time.
Figure 1 shows two foams, one prepared using the aromatic diisocyanate TDI, and another prepared using the aliphatic diisocyanate IPDI. These two foams were UV irradiated for 48 hours, and the bottom foam based on TDI exhibits a significant amount of yellowing. Thus, in order to choose the correct isocyanate, one must consider not only the cost, but the exposure conditions that will be observed by the coating, elastomer or sealant.
A handful of available diisocyanates are offered within the aromatic world, though the most widely used are undoubtedly TDI and MDI. Several ADIs are also available to the polyurethane formulator; of these, the most widely used are hexamethylene diisocyanate (HDI), isophorone diisocyanate (IPDI), and 4,4’-methylene dicyclohexyl diisocyanate or “hydrogenated MDI” (H12MDI). Despite the niche market that all ADIs maintain in the polyurethane world, some specialty ADIs exist even within those applications consisting of TMXDI (meta), TMDI, 1,3-HXDI and DDI.
One important note regarding the structure of ADIs to their individual inherent performances is whether they are cycloaliphatic (such as IPDI, H12MDI, TMXDI, 1,3-HXDI) or linear aliphatic (such as HDI or TMDI). When comparing performances based on one polyol, polyurethanes incorporating linear ADIs are typically softer and more flexible than those incorporating cycloaliphatic diisocyanates; conversely, cycloaliphatic diisocyanates impart rigidity and strength. However, polyurethanes containing a cycloaliphatic diisocyanate may still be flexible if a flexible polyol is used as its reactive partner.
Another performance variable is the structure of the diisocyanate. In ADIs, pendant groups can disrupt symmetry, which prevents crystalline segments from forming due to steric hindrances. All of the aforementioned ADIs are liquid, some of which only due to the mixture of the isomers within the product. As the polyurethane reaction begins, the urethane segments align and begin to hydrogen bond, forming hard segments. However, certain ADIs have structures that do not allow them to align perfectly to allow these hard segments to easily form.
Table 1 compares the reaction of four ADIs in a 1 molar:2 molar ratio with n-octanol in a 50% solution at 20°C. The structures of HDI and H12MDI allow them to begin crystallization during the reaction progress, though crystallization is not observed in either IPDI or TMDI. The crystallization of H12MDI in Table 1 makes it ideal for thermoplastic polyurethanes, as these hard segments will impart elastomeric properties in the final material. It is the crystallizable nature of the H12MDI-based TPU that will allow for physical property buildup without the need for further reaction (e.g., crosslinking), and can allow it to be processed and formed into various injection molded parts (see Table 2).
Conversely, reactive systems such as cast elastomers, reaction injection molded (RIM) systems, and foams can use a variety of ADIs. These systems often require good compatibility and a good price/performance ratio, which is often fulfilled by IPDI and, to some extent, H12MDI and HDI-based polyisocyanates.
Thus, a wide array of performance variables can be chosen when preparing polyurethane formulations based on ADIs. Nevertheless, the application must be considered when choosing the appropriate ADI and its polyol partner.
H12MDI VS. MDI IN THERMOPLASTIC POLYURETHANES
MDI and H12MDI have their roots in a cyclic structure with two isocyanate groups that are connected to the rings. It is the similarity in their chemical structures that will allow a comparison of the relative properties of their reactivity and their respective TPUs. Although the structures of MDI and H12MDI are quite similar, their three-dimensional structure needs to be taken in account. The MDI is nearly flat, while the cycloaliphatic structures have both trans- and cis-isomers with each ring. The cyclohexane rings themselves have a 3-D chair-like structure as well.
In addition to the isomer differences, certain electronic differences between MDI and H12MDI can affect reactivity, as shown in Figure 2. MDI and H12MDI were respectively reacted with n-octanol in a 55% solution of MOP-Ac at 80°C, and the conversion of the diisocyanates to dioctyldiurethane was monitored over time. Figure 2 shows the dramatically reduced reaction rate of n-octanol with H12MDI compared to MDI at 80°C. Despite the difference in reaction rate, the reaction of H12MDI can be catalyzed with only 0.01% DBTDL to reach the same conversion profile as MDI. Thus, despite the electronic differences between MDI and H12MDI, the reactivity difference can be compensated with a minor amount of catalyst.
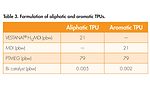
The next task was to determine the property differences between MDI and H12MDI in the development of a TPU. Table 3 shows the formulation of a TPU using H12MDI and MDI and PTMEG 1000, plus a minor amount of bismuth catalyst. These formulations were prepared via a batch process with a reaction temperature of 100°C, stirred for 30 minutes and then discharged. At this point, the NCO content ranged between 0.1-0.5 wt%. The formulations were then further stored at 70°C for two weeks to ensure a complete reaction.
The properties of the initial H12MDI and MDI TPUs prepared are shown in the first two columns of Table 4. Tensile measurements have shown that MDI using pure PTMEG 1000 as a polyol had not only a significantly higher tensile strength, but also a slightly higher elongation. Shore A hardness was also somewhat higher with MDI, and the chemical resistances measured against water, ethanol, and mineral spirits were improved using MDI. The principal advantage of H12MDI in the initial test was the lower yellowness index increase after 48 hours of UV exposure, which was remarkably reduced over that of MDI.
Due to the flatter and more rigid structure of MDI over H12MDI, a portion (8%) of the PTMEG 1000 was substituted with butanediol to reduce the flexibility on the polyol side and compensate for the increased flexibility from the crosslinker. The effect of this amount of polyol substitution is shown in the third column of Table 4: significant increase in tensile strength, hardness and chemical resistances without a detrimental effect to yellowness index. While the elongation could likely be increased with decreased amounts of butanediol, this example shows that a UV stable TPU can be prepared using H12MDI and still maintain—and possibly even improve—the properties of the TPU when using MDI.
H12MDI POLYCARBODIIMIDES
Various aliphatic diisocyanates such as IPDI and HDI can undergo advancement reactions to form structures such as isocyanurates, allophanates, uretdiones and biurets. H12MDI is one of the few exceptions that cannot easily be catalyzed to form some of these structures; however, it can be made to form a very interesting derivative of isocyanates (i.e., carbodiimides, specifically polycarbodiimides). Carbodiimides react with acid groups to form N-acyl-urea bonds. When a polycarbodiimide (PCDI) is present with acid groups, the PCDI can react with multiple acid groups, creating crosslinks.
In this work, H12MDI polycarbodiimides were prepared and used to determine if they could stabilize polyesters from decomposing in high humidity conditions. In the presence of high temperature and high humidity, polyesters can hydrolyze and, in the presence of PCDIs, the PCDI can then react with the exposed carboxyl groups. In this manner, the polyester would see no effective decomposition, as the PCDI would tie the broken pieces of the polymer together and the molecular weight would be maintained.
In this study, a polyester that is known to be unstable under hydrolysis conditions was chosen and then extruded with and without 1% of the H12MDI PCDI. These polymers were then exposed to elevated temperatures and humidity conditions over a period of eight weeks. They were intermittently tested for both viscosity number and acid value. A reduction in viscosity, as well as an increase in the acid value, would indicate a reduction in the molecular weight of the polyester. Specifically, the viscosity numbers that are shown were determined using a Ubbelohde viscometer with a 0.5% solution of polyester in cresol.
Figure 3 shows the effect of the storage of the polyester at 50°C under dry conditions. Under these conditions, the polyester shows a slight reduction in the viscosity number over a period of eight weeks without PCDI; adding only 1% PCDI prevented this drop in viscosity. The acid value, which increased slightly higher without PCDI, remains stable with the addition of PCDI. Furthermore, extrusion of the polyester causes some degradation, observed in the decrease in viscosity number and increase in the acid number from the initial polyester values. The addition of PCDI not only prevents molecular weight reduction in the extruder, but also builds the molecular weight to a small degree, as observed by the slight increase in viscosity number and decrease in acid value over the initial polyester values.
When this polyester (with and without PCDI) was exposed to 50°C and 90% humidity, a marked decrease in viscosity was noted in the polyester without PCDI after a period of three weeks (see Figure 4). With the addition of 1% PCDI, the viscosity decreased, but not to the extent that occurred without the PCDI addition. The acid value responded similarly, with a dramatic increase without PCDI but to a reduced degree with 1% PCDI addition.
Upon further rigorous testing, this polyester was exposed to 70°C and 90% humidity for a period of eight weeks. As shown in Figure 5, the polyester that had no PCDI incorporation showed a detrimental decrease in viscosity and a dramatic increase in acid value after eight weeks. With only the incorporation of 1% PCDI, the polyester showed a resistance to decomposition, both in viscosity reduction and in acid value generation. While the effect was not complete prevention of degradation, the incorporation of higher amounts of PCDI could provide additional resistance to decomposition. Furthermore, a traditional polyester (i.e., one with better hydrolytic stability) could see more benefit to the addition of PCDI than was observed in this study.
CONCLUSION
While aromatic polyurethanes play a dominant role in many global elastomer, sealant and adhesive applications, it is possible and perhaps necessary to substitute the aromatic diisocyanates by aliphatic diisocyanates to compensate for an increased demand for outdoor durability. While the substitution of MDI-based polyurethanes for H12MDI-based polyurethanes may not be direct, it may only require slight changes in catalyst and polyol composition to offer polyurethanes with similar physical properties plus outdoor durability. In addition, opportunities exist for polyisocyanates (particularly polycarbodiimides) in which diisocyanates could offer opportunities for growth as an additive and, as in the included example, specifically provide a unique opportunity for increased polyester durability.
For more information, contact the lead author at (973) 929-8904 or corey.king@evonik.com, or visit www.evonik.com/northamerica.
REFERENCE
1. “Diisocyanates and Polyisocyanates,” CEH Marketing Research Report (SRI), 2009.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!














