Q&A About Polyurethanes
Can existing formulations help make the leap from a one-component polyurethane coating to a two-component system?
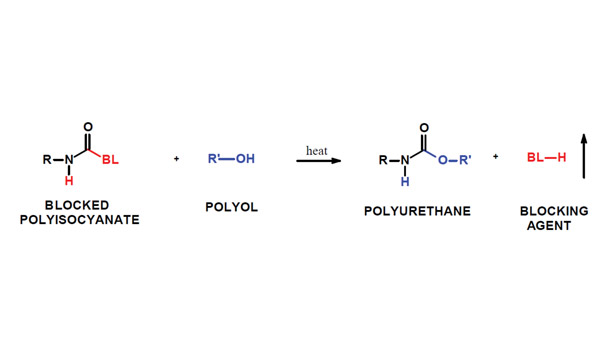
In many applications, the highest level of performance is achieved using two-component polyurethane systems. This is the frequently the case for solvent-based and waterborne coatings in addition to adhesives. However, one-component formulations are often preferred, since handling is simpler and it is not necessary to monitor mixing ratios of the isocyanate and polyol. The performance of a two-component coating, on the other hand, will suffer if the formulation is significantly off ratio. For example, if the isocyanate/polyol ratio is under-indexed—meaning there are more equivalents of polyol than isocyanate—the polymer will not achieve the required molecular weight, and properties will be unacceptable.
Blocked isocyanate chemistry may meet your needs for a higher performing, one-component coating formulation. This coating is formulated by mixing a polyol with a blocked isocyanate to produce a blend with room temperature stability. Upon heating, a high-performance coating is obtained:
Blocked isocyanates are produced through the reaction of an isocyanate, which can be aliphatic or aromatic, with a “blocking agent.” Typical blocking agents include caprolactam, methyl ethyl ketoxime (MEKO) 1,2-pyrazole, 1,2,4-triazole, 3,5-dimethyl pyrazole and diisopropyl amine. These blocked isocyanates are stable at room temperature. At an elevated temperature, the blocking agent is released, and the polyol reacts with the “free” isocyanate. Some blocking agents are volatile and are released from the film while others remain.
From a handling standpoint, these formulated coatings are processed as a one-component system. From a chemistry standpoint, however, the formulation is a two-component reaction of an isocyanate and a polyol. This reaction produces coatings that have performance characteristics comparable to standard two-component formulations but do not have pot life or mix ratio issues. The mix ratio is fixed when the coating formulator blends the blocked isocyanate with the polyol. Blocked isocyanates are available for solvent-based, waterborne and powder applications.
The temperature required to initiate the curing reaction varies primarily with the blocking agent used. Caprolactam has a relatively high deblocking temperature (~170°C), while diisopropyl amine deblocks at ~130°C. The time required to achieve complete crosslinking is dependent on the temperature in the curing oven.
Using MEKO as an example, at a temperature of ~170°C, the cure time is ~10 minutes, while at ~135°C the time increases to approximately 50 minutes. The curing temperatures can be considerably reduced by adding a small amount of a tin catalyst.
The high temperatures required to cure these formulations typically limit their use to substrates with high thermal stability, such as metal and glass. Blocked isocyanates formulations are well-established for metal applications, such a coil coatings, can coatings and automotive finishes. Both primers and top coats can be made. These coatings are valued for their excellent adhesion to steel, aluminum and galvanized steel, and formulations can be targeted to provide high flexibility and durability.
New products and applications are being developed using the basic blocked isocyanate chemistry. A Food and Drug Administration (FDA)-compliant blocked isocyanate for interior can coatings is available, and blocked isocyanates for textile coatings are also being explored. I encourage you to investigate blocked isocyanate chemistry if your application can tolerate the deblocking temperatures necessary to cure these formulations.
Any views or opinions expressed in this column are those of the author and do not represent those of ASI, its staff, Editorial Advisory Board or BNP Media.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





