"Boring" Boron and Adhesives
Boron plays an important role in the formulation of adhesives.







Considering the massive market size of adhesives and their importance in almost all sectors of materials and applications, boron likely plays a small yet definitive role in the adhesives industry. Unfortunately, recent peevishness over its unconfirmed toxicological issues poses a serious threat to the use of boron in adhesives. Regardless of the controversy, boron’s presence can cause significant changes in material properties in even small quantities. The seemingly innocent (some could say “boring”) element placed between beryllium and carbon has impacted our lives through its presence from agriculture to aerospace, from “Mummies to Rockets and onto Cancer Therapy,” as it was effectively addressed elsewhere.1
The association of boron compounds with human civilization dates back thousands of years. The name boron is most likely coined from the Turkish word for borax, “bor.” Boron as an element was unknown until it was isolated by Sir Humphrey Davy and Joseph-Louis Gay-Lussac in 1808. Thousands of years ago, boron minerals, known as “bor” or borax, were traded via silk routes using sheep, camel, and yak caravans to transport borax from Tibet to Egypt, Mesopotamia, and India, mainly for gold jewelry, preservatives, and medicinal purposes.
The Egyptian mummification process hinged on using an ore known as Natron, which contained borates as well as some other common salts. Its use is mostly lost in antiquity, and it is not clear whether borax ores were used for the preservation of the mummies, for improving the bonding of the plastering cloth around mummies, or both. In the tombs and mummies of King Tut and other Egyptian pharaohs, animal- and plant-based glues were used for lamination and bonding. Following the archeological findings, it won’t be too farfetched to assume that boron most probably had a major role in the adhesion aspects of the mummifying process, in addition to its biocidal properties for preservation.
Unique Element
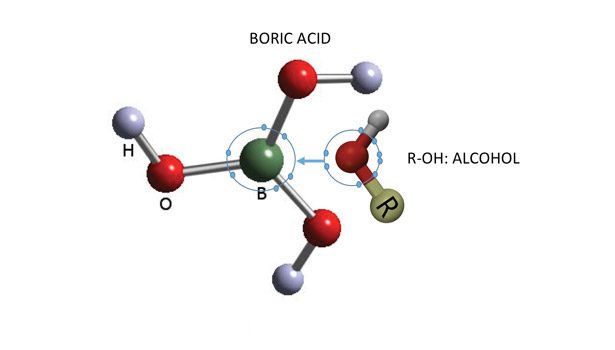
Before going into the details of boron-based adhesives, we must address a question: What is there in boron that makes it so unique? As the first element of the third group (1s2, 2p1) in the periodic table, boron has a smaller atomic radius. When boron forms a compound, it is characteristically electron deficient. To be more precise, boron forms a compound with an incomplete octet of electrons around the central boron atom (see Figure 1).
This deficiency makes boron compounds interesting; it makes a boron compound a strong Lewis acid (i.e., it makes boron extremely hungry for a pair of electrons to complete the octet). As a result, any chemical group, moiety or radical that is capable of sharing a lone pair of electrons (Lewis-base type) to a boron compound with an incomplete octet, undergoes a Lewis acid-Lewis base type of reaction to complete the octet. Compounds containing oxy, hydroxy, amino, chloro, etc., functional groups are very good examples of Lewis base in that respect.
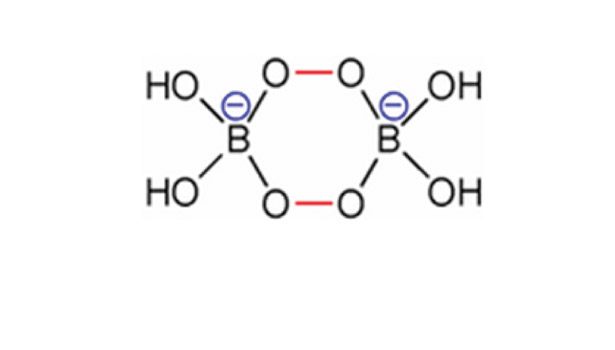
Although boron compounds are widely distributed in the Earth’s crust, their abundance in concentrated forms is few. Turkey has the lion’s share of boron deposits—around 72% of the known global reserve. Alkali and alkaline-earth metal borates are the most commonly found ores on Earth. As mentioned, boron’s hunger for a lone pair of electrons provides myriad possibilities to produce a variety of compounds and molecular complexes. For example, when a solution of borax and hydrogen peroxide is crystallized, sodium perborate (NaBO3 * 4 H2O) is formed. Sodium perborate is used in color-safe bleaches. The key to the bleaching ability of this compound is the presence of its two peroxo groups that bridge the boron atoms together (see Figure 2), following a similar mechanism as shown in Figure 1.
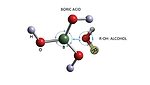
The most important compound that other boron compounds can be synthesized from is boric acid B(OH)3 (see Figure 1). When mixed with water, the weakly acidic and electron deficient boric acid accepts an OH- ion from water and forms the complex ion [B(OH)4]-. The strong affinity for a pair of electrons in boric acid and other borate compounds makes them valuable for adhesive applications like many other fine chemicals.
Stereo-chemically, borates can be of both tetrahedral and trigonal planar (e.g., boric acid) configurations. Borates in the presence of water dissociate as meta-borates and boric acid:
B4O72-(aq) + H2O(l) HB4O7-(aq) + OH-(aq)
B4O72-(aq) + 5H2O(l) + 2H+(aq) 4H3BO3 (aq)
B(OH)3 (aq) + H2O(l) B(OH)4-(aq) + H+(aq)
As one may realize, boric acid’s acidity is due to the acceptance of an electron pair rather than by proton dissociation. Boric acid is therefore mono-protic, not tri-protic as expected.
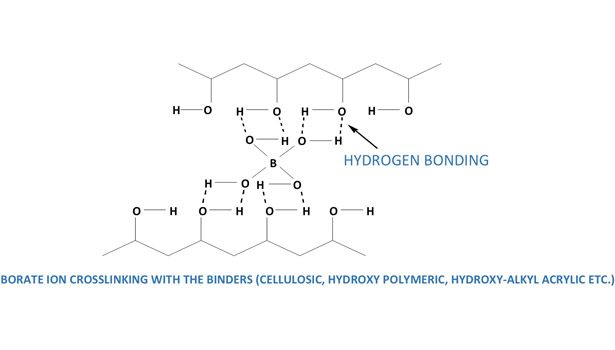
The tetrahedral B(OH)4- undergoes bridging and gels with any polyol, cellulose, polysaccharide, glycoprotein, etc. The interaction of simple carbohydrates and borates is well known. After interacting in the presence of borate, a neutral polysaccharide becomes negatively charged.2 Figure 3 illustrates how boron in boric acid and borates form a hydrogen bond network with a compound containing poly-hydroxy groups. This is the basis for its importance in adhesives.
It is important to remember that the critical importance for adhesion to a substrate is the wetting and the surface force. The most important aspects to consider in terms of the molecular structure of the adhesive include the chain length and the presence of any branched chains, the presence of polar groups, and viscosity. The presence of polar groups in an adhesive greatly impacts the surface forces.
High viscosities are always preferred in order to prevent over-running of the adhesive at the edges of the bonded joints. The presence of borates or boric acid in waterborne adhesive formulations increases the viscosity by increasing the molecular weight of starch or poly saccharides, casein, glycoproteins, and polyol types of molecules after forming complexes with their hydroxyl (-OH) linkages. Viscosity can be altered to a desired extent by varying the concentration of boron, depending upon the application requirements.
Starch-Based Adhesives
Starch-based adhesives are used in paper bonding and paper products such as bookbinding, corrugated boxes, paper bags, and wallpaper pastes. Carbohydrates extracted from vegetable plants such as corn, rice, wheat, and potatoes are generally used for such applications. Borax (sodium tetraborate decahydrate) and sodium metaborate (essentially a mixture of borax and sodium hydroxide) dramatically change the properties of cooked starch. Increasing borax content increases viscosity; tack property and cohesiveness are also greatly increased by the addition of borax.
Borax can be used as high as 10% based on the type of starch used. Borax is generally added prior to cooking the starch. Borax forms complexes with the starch molecules following the same basic mechanism, as shown in Figure 3. The boron starch complexes are negatively charged, and they can also further crosslink to form higher molecular weight starch complexes causing a large increase in viscosity. Depending on the application, starch-based adhesives can be both cold- and warm-water miscible.
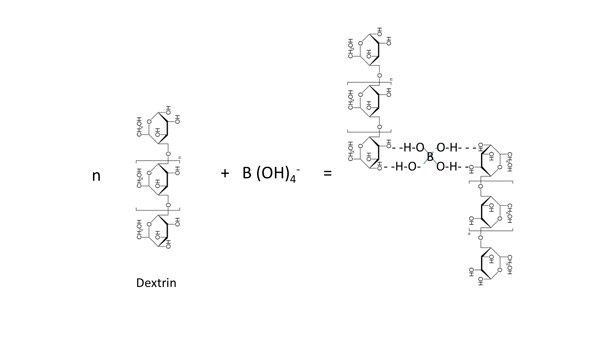
Dextrin is frequently used along with starch in various waterborne adhesives to provide improved tack and higher viscosity with stable rheology at moderate concentrations. The use of any form of borate (e.g., borax, sodium metaborate or boric acid) in combination with sodium hydroxide in dextrin-starch based formulations provides very good tack property. The formation mechanism of a borated starch-dextrin complex is shown in Figure 4. These types of adhesives are mainly used for case sealing, carton sealing, tube winding and laminating.
Borates in adhesives made from any cellulosic materials function in a similar manner by bridging through a hydrogen bond network, as shown in Figure 4. Typical formulations for starch-dextrin based adhesives involve cooking water (50 wt%), white starch-dextrin (35 wt%), preservative (1%), borax (6%), and an antifoam (0.05%) at high temperature for 20 min. The cooled mixture is further diluted with water (7.5 wt%) and 12N sodium hydroxide (0.5 wt%).
The addition of alkali increases the thickening effect and markedly improves the tack property of the starch adhesives. Alkali helps borax to hydrolyze into the monoborate ion [B(OH)4-] that partakes in complex formation with the starch molecules.4,5 However, it must also be noted that an excessive use of alkali and borax can have a detrimental effect on the adhesive property, as they can sever the hydrogen-bonded network, causing a drop in viscosity and tack.
Animal Glue
As mentioned earlier, animal glue is one of the oldest-known adhesives. Animal glue is the water-miscible product made from the protein extracted from animal bones, hide, hoofs and horns by boiling in water. The extracted proteins undergo hydrolytic degradation of insoluble collagen fibers present in the protein molecules.
The extract is cooked to form a gelatinous material. Gelatin contains primarily the denatured form of Type-I collagen, a glycoprotein.3 Use of boric acid and borax in animal glues is mainly for rheology modification; high temperature tolerance; improved water resistance; and resistance toward fungal, bacterial, and insect attacks. Animal glues can be re-liquefied with heat, which gives them quick setting properties.
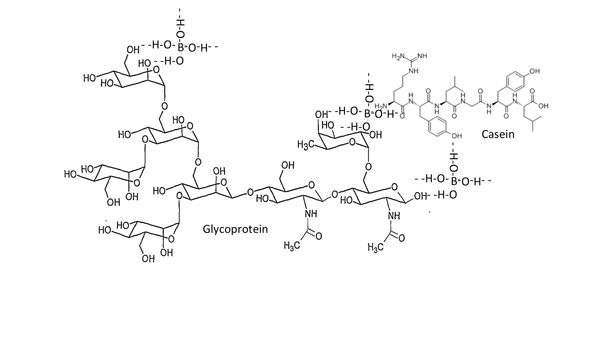
Major uses for animal glues have been in manufacturing wood products and furniture; animal glues for furniture are also often formulated with casein for improved adhesive property and mechanical strength. A schematic of the association of borate ions at one of the several sites present in the glycoprotein molecule in the presence of casein is shown in Figure 5.
Neutral plastic adhesive compositions are prepared from a composition comprising a coaction product of a resin formed by reaction of hydrochloric acid, tar, Congo gum, and urea, and cooking the said resin with an animal glue, casein, borax, sodium benzoate, oil of Wintergreen, and water.4,5 The addition of casein or any polysaccharide (e.g., dextrin, etc.) provides improved plasticity to the adhesive. In the presence of starch, dextrin or casein types of materials, glycoprotein molecules can bridge with them through the B(OH)4- ion by forming a massive H-bond network (see Figure 5).
Overall, the traditional art of making adhesives from either tree saps, starch, or animal extracts—whether used alone or in combination—always included borax to improve wet tack, stability, and durability. Borate ion’s fundamental role is to bridge those polymeric structures to a higher-molecular-weight, branched polymer complex.
A good example is the recent development of a soy protein-based adhesive. Soy protein’s unique functional characteristics are of very high value for the preparation of adhesives. A soy protein-based formulation involves the addition of boric acid with a pre-cooked soy powder, citric acid and sodium di-hydrogen phosphate (NaH2PO4). The addition of boric acid improves shear strength of the bonded samples when treated with water, indicating that boric acid improved the adhesion and water resistance via the formation of high-molecular-weight bridged complexes.
PolyVinyl Alcohol-Based Tile Adhesives
A compound of cellulose ether, polyvinyl alcohol and boric acid or its salts (preferably borax) is used as a thickener for tile adhesives. Due to the addition of boric acid or borax, these systems have an improved thickening action compared to the mixtures composed of cellulose ether and polyvinyl alcohol alone. Figure 3 illustrates the mechanism for how borate ions bridge with a poly-hydroxy compound similar to poly-vinyl alcohol.
Rubber-Based Contact Adhesives
Rubber-based adhesives are made by combining one or more rubbers or elastomers in a solvent. These solutions are further modified with additives to improve tack, peel strength, flexibility and viscosity. Rubber-based adhesives are used in a variety of applications, such as contact adhesives for plastic laminates like counter tops, cabinets, desks and tables. They are also used in pressure-sensitive tapes, floor tiles and carpets. Self-sealing envelopes and shipping containers also use rubber cements. Rubber adhesives have been the mainstay of the leather industry.
Because of increasing stringency toward the use of solvents in adhesives, however, water-based contact adhesive formulations are on the rise, where the use of boric acid happens to be a great choice for improved storage stability, set time, and tensile strength with high elongation characteristics. Such adhesives consist of a blend of poly-chloroprene latex and styrene-acrylate emulsion in an optimized boric acid solution with zinc oxide as a proton inhibitor. In addition to improving shelf life of the adhesive mix by preventing coagulation of latex, the use of boric acid also accelerates the formation of the bond of the poly-chloroprene and styrene-acrylate-based contact adhesives.6,7 A possible mechanism for the function of boric acid is shown in Figure 6.
These examples are just a few in which borax or boric acid directly interacted to form an adhesive matrix by aiding crosslinking of the active molecules. Nevertheless, there are various applications where boron compounds are used indirectly either for the synthesis or for some special effects in synthetic adhesives as well.
Synthetic Adhesives
The first synthetic polymer was cellulose nitrate, a thermoplastic material synthesized from wood cellulose that came on the market during the late 19th century. Soon thereafter, the era of plastics began accelerating to meet the growing demand for new materials. With the advent of plastics, the evolution of synthetic adhesives also started growing. The development of plastics and elastomers has rapidly advanced the development of adhesives and has given formulators a variety of products that can change and improve various properties of adhesives, such as flexibility, toughness, curing or setting time, temperature, and chemical resistance.
Synthetic adhesives are used predominantly in everything from furniture to airplanes. Synthetic adhesives can generally be organized into two groups: reactive and nonreactive. Nonreactive adhesives are of two types: solvent-based adhesives that get cured as the solvent evaporates (e.g., white glue, contact adhesives, rubber cement etc.); and polymer dispersion types, such as polyvinyl acetate (PVAc), which are extensively used in the woodworking and packaging industries. For waterborne nonreactive systems like rubber cements, the uses of boric acid or borax are already discussed. In waterborne PVAc adhesive systems, the ester groups of PVAc are sensitive to hydrolysis forming hydroxy components of the polymer.8 Using borax or boric acid under an alkaline condition causes the polymer to crosslink like polyvinyl alcohol does (see Figure 3).
Reactive adhesives mainly include urethanes, epoxies and cyanoacrylates. These adhesives harden through a chemical reaction. In some systems, curing takes place by mixing a two-part system; the first part comprises the polymers or resins, and the second part consists of a curing agent. In some adhesive systems, curing is carried out by exposing resins to heat or air. The areas where boron compounds are used in those reactive adhesive systems are briefly discussed below.
Urethane Adhesives
Urea-formaldehyde (UF) and phenol formaldehyde (PF) adhesives are primarily used in wood industries to make plywood, particleboard and medium-density fiberboard (MDF). The emission of formaldehyde is an important factor in the evaluation of the environmental and health effects of the wood-based board materials. Use of an optimized level of borax in the UF or PF adhesive formulations significantly reduces the formaldehyde emission without altering the mechanical strength.9 Boric acid is used in polyurethane-based (PU) wood glues for wood protection without compromising the bonding strength.10
Epoxies
Epoxy adhesives are made by a complex chemical reaction. Various resins are made synthetically by reacting two or more chemicals. Boron tri-fluoride is used as a cationic catalyst for homo-polymerization of epoxy resins. Homo-polymerized epoxies often need high-temperature curing. Epoxy adhesives can bond a variety of substrates (particularly metals) with high strength. They have been used to replace some traditional metalworking methods of joining like nuts and bolts, rivets, welding, crimping, brazing, and soldering.
Epoxy-based adhesives are ubiquitously used as interface materials for electronic packaging. The greatest advantage of epoxies is that adhesive formulators can have excellent formulation latitude and enable flexibility to custom design their formulations using fillers. Boron nitride-filled epoxies exhibit superior properties as thermal interface materials compared to silicones, greases, tapes, or pads. Two-component boron nitride epoxy adhesives are used for high-performance bonding, sealing, coating and potting. Boron nitride-based thermally conductive epoxies are lighter in density, which is particularly desirable for aerospace and defense applications, and for microelectronic assemblies.11-13
Hot-Melt Adhesives
Hot-melt adhesives are thermoplastic solid polymers liquefiable at elevated temperatures only. The major use of hot-melt adhesives is in carton sealing (e.g., frozen food packages, breakfast cereal boxes, laundry detergent packaging, beer cartons, etc.) where the carton folds are bonded with a hot-melt adhesive. They are also used residentially for fast repairs around the home.
In reactive hot-melt adhesives based on curable silicone resins, boron halides or boron halide complexes are used as curing agents. This type of fast-curing, reactive hot-melt adhesive hardens and stabilizes rapidly at around ambient temperature, resulting in improved application efficiency. Thus, they are very useful industrially.14,15 Tri-phenyl and tri-fluoro boranes are also used in the manufacture of copolymerized aromatic vinyl compounds (e.g., styrene and butadiene) for hot-melt adhesive applications.16
Cyanoacrylates
Cyanoacrylates are extremely rapidly curing adhesives known as superglues. They are typically used in applications where there is a need for a rapid curing, single-component adhesive that has high adhesion, high strength, and easy dispensing. Boron oxide and boron tri-fluoride complexes are used as acid inhibitors and polymerization inhibitors (or stabilizers), respectively, during the synthesis of cyanoacrylates.17-19
Silicone Adhesives
Boron oxide and boric acid are used as fillers in silicone adhesives. Organometallic derivatives of boronic acids are used as photo-initiators in UV-curable compositions. Boron nitride is used in thermally conductive silicone adhesives. Self-adhering silicon rubbers with improved surface properties are synthesized using boric acid (< 0.5 wt%) for the manufacture of pressure-sensitive adhesive elastomers. It is well known that the addition of boric acid to silicones improves adhesion to metal.20
Present Scenario
Boron’s use in adhesives shows that it plays primarily a structural crosslinking role, unless it is used in the form of a catalyst or as a nonreactive additive. In crosslinked adhesive products, borate ions are all bound with the adhesive matrix. In almost all applications, the maximum level of boron compound usage is below the stipulated limits set by the regulatory authorities (< 11 wt%).
The evidence of boron’s role in adhesives merits a critical reconsideration by the authorities to ease the fear regarding its usage. Raising an alarm against boron products based on the studies of limited relevance seems to be an over-reaction when there is no confirmation of boron being detrimental to human health. Regardless, concerns with health issues with boron compounds opened new vistas to search for substitutes to replace boron. In many cases, however, such hasty attempts to replace boron compounds are found to be no less damaging to environment and health, and often are costlier. For example, boron compounds are replaced by chlorine and enzymes in detergents; lithium compounds are used to replace borates for making enamels and glass products; ammonium sulphate/magnesium sulphate are used as an alternate to borate for fire retardation, etc.
Nonetheless, despite all founded or unfounded attempts to displace boron compounds from their use in the chemical industry, the boron compounds and boron ores, which have been known for centuries, may easily outlast those concerns and meet the demands for many coming years. The future of boron is only limited by our imagination and diligent scientific investigation.
For more information, contact the author at One Penn Center West, Ste. 400, Pittsburgh, Pa., 15276; (412) 809-8215 or arun@etimineusa.com; or visit www.etimineusa.com.
Acknowledgements
The author is immensely grateful to the management of Etimine USA Inc. for the encouragement to publish this article. The author is also grateful to his colleagues Philip Ford and Susan Zelicoff, and to his daughter Bidyunmala for their ungrudging assistance with the preparation of the manuscript.
References
1. Hosmanne, N. (ed), Boron Science and New Technologies, CRC Press, Taylor and Francis Group (2012).
2. Zittle, C.A., and Harris, T. N., J. Biol. Chem., 142, 823 (1942).
3. Klotz, Stepane A., and Smith, Robert L., Microbiology, Vol. 141, pp. 2681-84, 1995.
4. Hirsh, Eugene, U.S. Patent US2208580 A, July 3, 1940.
5. Rolando, T.E., Rapra Review Report, Vol. 9 (5), 1-20 (1995).
6. Varghese, L.A., and Thachil, E.T., J. Adhesion Sci. Technol., 18, 181 and 1217 (2004).
7. Zhang, K., Shen, H., Zhang, X., Lan, R., and Chen, H., J. Adhesion Sci. Technol., 23, 163(2009).
8. Staudinger, H., Frey, K., and Stark, W., Ber. Deut. Chem. Ges., 60, 1782 (1927).
9. Ozlap, M., European J. Wood and Wood Products, 69(3), 369 (2011).
10. Leser, B., Ugovsek, A., Kariz, M., Sernek, M., Humer, M., and Karlz, P., Wood Research, 56(3), 285(2011).
11. Reversibly Adhesive Thermal Interface, U.S. Patent Application 2012/0182693 A1.
12. Methods of Forming Bonded Semiconductor Structures; U.S. Patent Application 2013/0299997 A1.
13. Hodgin, Michael J., and Estes, Richard H., Proceedings of the Technical Programs, NEPCON WEST 1999 Conference, Feb. 23-25, pp. 359-366, Anaheim, Calif.
14. Reactive Hot Melt Resin Composition and Reactive Hot Melt Adhesive, Patent JP 01788035/EP-B1.
15. Fire Resistant Hot-Melt Adhesives Containing Modified Polyethylene, U.S. Patent 4440888 A.
16. Hot Melt Adhesive Composition Containing α-Olefin/Aromatic Vinyl Compound Random Copolymer, U.S. Patent 6235818 B1.
17. Adhesive Compositions for Medical Use: Single Additive as Both the Thickening Agent and the Accelerator, USPTO Patent Application U.S. 2012/0264846 A1.
18. Viscous Alpha-Cyanoacrylate Compositions, U.S. Patent Application 2009/0326095 A1.
19. Acid Chelate of Boric Acid or a Derivative Thereof with a Poly-Hydroxy Compound, U.S. Patent US4182823 A.
20. Acton, Ashton (ed.), Boron Compounds-Advances in Research and Application, Scholarly Edition (2013).
Historic Connection
Although adhesives have been known for about 6,000 years, most adhesive technology has been developed only during the last 100 years. The first evidence of using a substance for adhesion dates back to 4000 B.C. Archaeologists found prehistoric artifacts glued with sticky resins from tree saps. In Babylonian temples, the ivory eyeballs of the statues were glued into eye sockets with tar-like substances that lasted for almost 6,000 years.
During the period of 1-500 A.D., the Romans and Greeks mastered the art of veneering and marquetry. They developed refined glues from animal and fish blood, bones, and hide, as well as adhesives from egg whites and other natural ingredients such as milk, cheese, vegetables, and grains. After this, the development of adhesives remained dormant or not recorded until about 1500-1700 A.D., when the use of a specific substance called adhesive became part and parcel of the furniture industries. After 1700 A.D., particularly after the industrial revolution, rapid changes began in developing and understanding adhesives.
Irrespective of the historical facts of the adhesives used, which were mostly plant-, animal- and starch-based, one product that was most commonly found in many instances was borax or some boron forms, such as ores. The addition of borax or related materials was primarily used for preservation without realizing their roles in improving the properties of glue. Many studies in recent times only confirmed the historic fact that the addition of boron compounds to the glue did not have any negative impact on the performance of the glue. On the contrary, some properties were even improved.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!












