Achieving Adhesion to Difficult Substrates
A new material can be used to improve adhesion in a variety of substrates.










Adhesion, or lack of it, is one of the most critical attributes for most adhesives, coatings and inks. A variety of options can be explored when it comes to adhesion. Two of the most common types of adhesion are mechanical and chemical. Mechanical adhesion occurs when adhesive materials physically lock into the crevices of the surface. This requires that the substrate surface be physically abraded or chemically etched, unless the substrate has a naturally rough surface.
Chemical adhesion can be improved through the use of coupling agents, such as silane acrylates, or through under curing a primer coating. When improved adhesion was recently required in several different UV-curable applications, a specific material was developed that could be used for a variety of applications.
Strategy
Visible-light-cured adhesives have been an essential part of light-cured dental systems since the technology was first accepted. Generally speaking, light-cured dental adhesion promoters must retain their adhesion in challenging environments, such as the mouth. They must also be effective to a range of substrates (e.g., titanium, bone, glass, etc.).
After examining several adhesion promoters used in the dental industry, it was determined that most of these materials have similar functionality. In many cases, the molecule has a carboxylic acid group attached to the molecule. This group is important in achieving the desired bonding to the surface. It is also necessary to get the molecule to crosslink with the rest of the dental formulation. To achieve this crosslinking, a methacrylate group is another part of the parent structure. As the desired target in this study was a relatively low molecular weight, low-viscosity monomer to facilitate blending with the rest of the formulation, the carboxylic acid and (meth)acrylate group was viewed as essential, with the rest of the molecule being kept as small as possible to avoid any undesirable properties.
The basic strategy can be seen in Figure 1. In essence, a polar group A is grafted onto either a methacrylate or acrylate molecule to result in an adhesion promoter C. The R and R1 groups were varied in this experimental work.
Test Results
A series of potential adhesion promoters were synthesized by varying the polar group and attaching either methacrylate or acrylate functionality. These materials have been given the product code PL-2XXX. In addition to the base structure C, an adhesion promoter with phosphate functionality (PL-2110) and an acrylic acrylate (PL-2317) were also prepared and tested along with the targeted series.
In the first round of evaluations, a mixture comprising 97% adhesion promoter and 3% photoinitiator were mixed, drawn down on the test substrate and then cured under UV light. Once the sample was cured, they were tested using the typical cross hatch test (ASTM D3359-09). The results for a series of metal substrates can be seen in Table 1. For some of the test substrates, wetting out of the simple formulation was unsuccessful, thus resulting in samples that were insufficient to test. These are labeled NA in the Table 1.
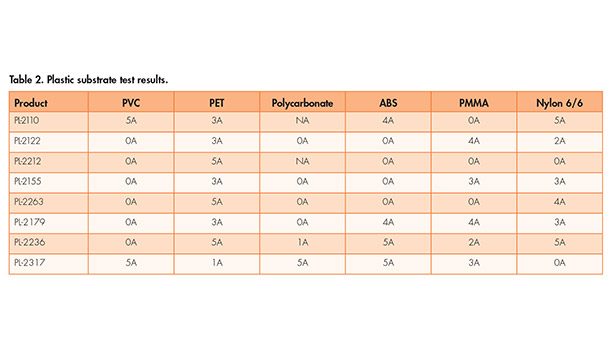
In a typical end-use formulation, additives would usually be incorporated to help promote wetting of the substrate, therefore eliminating this type of failure. With the success seen on metal substrates, the program was extended to an evaluation of the adhesion promoters on different plastic substrates. These results are shown in Table 2. Once again, NA is used to indicate when a wetting out problem occurred with the pure adhesion promoter in this evaluation.
There are two common plastic substrates that are missing in Table 2. The series of adhesion promoters was tested on both untreated polyethylene and polypropylene. In all cases, good adhesion results were not obtained on these two substrates. When formulating coatings for either PE or PP, the typical approach to pre-treat the substrate with a technique such as corona, flame or plasma is usually required.
When approaching the selection of the type of UV-curable group that would work the best for adhesion promotion, many formulators will show a preference for either a methacrylate or acrylate depending on their previous experience. This study presented the opportunity to compare the effect of either an acrylate or methacrylate on adhesion when the rest of the molecule remains the same. In this study, PL-2122 and PL-2212 share the same polar group A (see Figure 1) but differ in that PL-2122 is a methacrylate and PL-2212 is an acrylate. In the same vein, PL-2155 is the methacrylate equivalent of PL-2263. There appears to be no consistent advantage in using an acrylate or a methacrylate group when it comes to optimizing the adhesion characteristics of the adhesion promoter.
Glass is a challenging substrate to adhere to, yet can be found in a range of UV-curable applications. An assortment of adhesion promoters were evaluated on clean float (window) glass. As can be seen in Table 3, it is possible to achieve a high level of adhesion when using a basic structure such as Adhesion Promoter C.
The test results reported so far in this article were based on a system using only the adhesion promoter as the UV-curable material. In almost all practical formulations, there is usually a mixture of UV-curable products used to achieve the desired end product performance. While a material may perform one way when it is the sole component in a formulation, different results may be obtained when it is part of a mixture. The performance of one of the adhesion promoters (PL-2121) blended in with an aliphatic urethane diacrylate (Exothane 26) was evaluated. As typical levels of adhesion promoters in formulations tend to be less than 10%, a range of blends were made at various levels of PL-2121 in Exothane 26. The photoinitiator used was PL-450 at a 3% level. The results are reported in Table 4.
The base Exothane 26 can be seen to have minimum adhesion when used as the only component (0% PL-2121). This is typical for many aliphatic urethane diacrylates. As expected, as the amount of PL-2121 was added to the formulation, the adhesion increases accordingly. While these results matched expectations, the test results are highly dependent on the combination of resin components, adhesion promoters and substrates. Additional work is needed to be able to predict with a high degree of accuracy the level of adhesion with any particular combination of materials and substrates.
Improving Adhesion
Based on results seen in the dental market, a strategy was developed to target a relatively low-molecular-weight, low-viscosity adhesion promoter for industrial applications. A series of molecules was prepared by varying the energy-curable group (acrylate and methacrylate) and the polar functionality to promote adhesion. These materials were evaluated on a wide range of substrates ranging from metals to plastics to glass.
It was demonstrated that it is possible to significantly improve adhesion to challenging substrates using variations of the generic adhesion promoter structure C. Simple test formulations have shown that the appropriate adhesion promoter will improve adhesion when blended in a typical range of 2-10%. These lab results have been confirmed through several recent commercial applications.
For more information, contact the author at (610) 442-6589 or mike.idacavage@esstechinc.com, or visit www.esstechinc.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!











