Two-Part Adhesives and Application Equipment
Many different starting materials are available in the formulation of two-part adhesives, all of which will require a variety of mixing, dispensing, and application methods.



Two-part adhesives are an ever-growing segment of the adhesive and sealant industry, with applications in aerospace, construction, automotive, consumer goods, and the DIY/repair sectors. With the U.S. accounting for roughly one-third of the adhesives used worldwide, demand for cost-effective, efficient products that meet the desired criteria—formulating, mixing, metering, and dispensing—are all critical components that must be identified and optimized to ensure that quality and quantity demands are met.
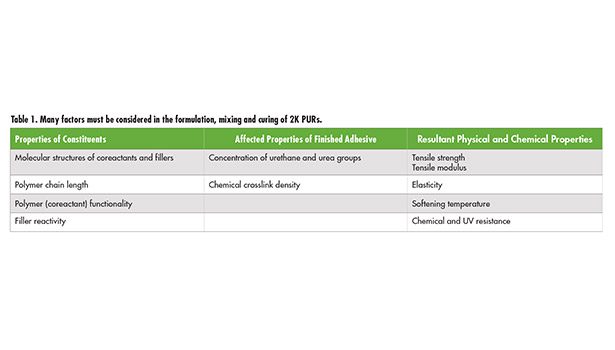
Many different starting materials are available in the formulation of two-part adhesives, all of which will require a variety of mixing, dispensing and application methods. A discussion of such breadth and depth is covered in the wide assortment of texts,1 with this article focusing primarily on two-part (2K) polyurethane (PUR) reactive adhesives, which cure at room temperature, and the mixing conditions to consider when selecting equipment. Table 1 provides a brief summary of various properties that should be considered throughout the numerous steps in the manufacture of a 2K PUR system.
Ambient-Cure 2K PUR Adhesives
2K PUR adhesives, which cure at ambient conditions, are comprised of: 1) polyisocyanates that are either low-molecular-weight or that can be pre-reacted and 2) a low-molecular-weight polyol component. This variety of reactive PUR adhesive is ideal when good heat stability of the joint is required, and is achieved through chemical crosslinking of the polyol and isocyanate through a polyaddition reaction, which forms urethane groups.1
The most visible use for PUR adhesives is two-part systems that have brief working times followed by a curing period, during which the two components crosslink chemically or react with one another to provide the finished material. It is the combining of these two components that produces the physical properties of the finished product and the strength of the bond. The ratio of each component used can be thought of like a recipe; for example, where varying ratios of water, flour, and additives are combined and how they are allowed to “cook” together results in food products as varied as bread, pasta, and cake.
The need for stoichiometric quantities of each component is apparent; the molecules react with one another in specific ratios, and if there is too much or too little of any given component, the quality of the joint will be affected. Isocyanate should be on the order of approximately 10% excess above the stoichiometric ratio required to complete the reaction. The reason for this excess is to ensure adequate crosslinking is achieved even in sub-ideal environments (e.g., residual moisture or environmental impurities on substrate surfaces, inadequate homogenization of the two components, etc.). This ensures that the bond will be strong enough to effectively transfer energy through the complex material and thereby perform its function.
Much effort, energy, money and time goes into formulating a new product—and rightly so, as there is potential for great gains when expectations are met or exceeded. The chemical and physical interplay of components in a formulation is given great consideration, as well as the physical requirements of the mixing and dispersing equipment to execute the goal. However, these are viewed as discrete entities (or areas of project management) that overlap little, if ever. The very nature of the constituents of 2K PURs results in mixing considerations that many will overlook and few—if any—outside of the dispersion equipment industry are aware of such obstacles, or the equipment available to remedy such impedances. When one considers not only the mixing requirements of each component (or the mixing requirement of the two parts combined) but also how the components and parts behave in contact with the mixing equipment, a more complete picture is formed, largely due to interface phenomena.
High-surface-energy materials are solids with a surface energy greater than 37 dynes/cm (dyn/cm = 10-3 N/m). Liquids “spread” to a greater extent on solids with a high surface energy; that is, the liquid will exhibit a decreased contact angle on a solid of high surface energy vs. a solid of low surface energy. The contact angle can be found through experimentation, or through use of the Young’s equation when the surface energy (or surface tension, for liquids and gases) for all moieties is known.
Pioneering work by William Zisman, Ph.D., in the areas of wetting, surface tension, adhesion and contact angle aided the understanding and interconnectivity of these topics. What we glean from Zisman’s work is: “A surface is more wettable when YLV [the surface tension between the liquid and vapor] is low and when U is low. He termed the intercept of these lines when the cos U = 1 as the critical surface tension YC of that surface. This critical surface tension is an important parameter because it is a characteristic of only the solid.”2 In addition, the majority of molecular liquids achieve complete wetting (U = 0°) with high-energy surfaces such as stainless steel, which has a surface energy range of 700 to 1100 dyn/cm.
Due to the surface energy, contact angle, and surface (interfacial) tension between the solid (stainless steel), liquid (A or B part of 2K PUR adhesive), and the vapor phase (air, vacuum, or vapor caused by volatilization of the A/B components), the cleanup process between batches is costly. Time that the disperser is not in use for cleaning lowers plant productivity, and due to the high surface energy of stainless steel, it is highly wettable, and thus more difficult to clean (where cleanability is technically defined as the inverse of wettability).
Dispersing 2K PUR Adhesives
For high-quality (very low contamination levels) 2K PURs, even the smallest of contaminations could pose a threat to the finished products’ quality. To ensure that cross-contamination is virtually eliminated, a dispersion system was developed with interlocking removable shafts, bearing housings, tank cover and mixing vessel. This system eliminates batch-to-batch contamination while increasing disperser productivity by reducing downtime between batches. In addition, the system can reduce capital demand by requiring less disperser floor space, which also enables even smaller facilities to produce the desired amount of product.
With reduced potential instances for human error in the dispersion process and a streamlined, simplified start-to-finish process, working costs can also be reduced. Reduced machine cleaning time (mixing vessel, tank lid, shafts, blades, etc.) reduces labor costs while improving the efficiency of each dispersing unit and simultaneously increasing the plant’s productivity. A rugged change-shaft/change-can style disperser was designed for continuous use, with periodic maintenance comprising the majority of a small non-operational fraction of gross potential working time.
Because two-part adhesives are often sold in small individual amounts, one batch each of the A and B component produces many units for purchase by consumers. For this reason, a smaller company or product line may not warrant the purchase of two autonomous mixers—one for the A part and one for the B part. Conversely, using one standard mixer will result in either producing multiple batches of one part of the formulation, or producing in an A-B-A-B sequence, which will have product ready for shipment in less time after production, but will result in significantly more downtime as the machine is thoroughly cleaned between each batch.
As previously discussed, stainless steel—used to manufacture the majority of “clean” surfaces in mixing vessels—has a relatively high surface energy, and liquids tend to spread (i.e., wet) on surfaces with high surface energy. As a result, the contact angle the liquid formed with the stainless steel is reduced when compared to a solid of low surface energy, and is said to be wettable, or have higher wettability than a low-surface-energy solid. Due to the fact that the physical interplay between the adhesive components and the stainless steel promotes spreading wetting on the stainless steel surface, it may be more difficult to fully remove all contamination from the surface. This is explained by the fact that wettability and cleanability are properties of a material that are inversely related through contact angle, surface energy, and the critical surface tension.
Mixing 2K PUR Adhesives
Viscous products that do not flow under their own gravity can present challenges during product discharge and packaging. Traditionally, viscous, non-flowing and/or shear thickening fluids are removed from the mixing vessel with the assistance of a ram press. A basic ram press design consists of a depressible plate, which extrudes the product through a valve at the bottom of the mixing vessel. With a thick product (e.g., one that “holds peaks,” similar to whipped cream), removal of the shafts will create a void in the product. This can result in an entrapped pocket of air, which may be forcefully expelled from the vessel as the ram press advances—a result of the air compressing when pressure is applied and expanding in volume upon approaching the exit valve or contact with the atmosphere. This not only results in potential time delays due to cleanup, but also due to improperly filled product cartridges.
Many options are available to modify a ram press for a specific application; for viscous 2K PURs, a vacuum-assisted ram press minimizes cleanup, product waste and cartridge filling errors. By using a vacuum-equipped ram press, many cartridge filler and product consistency issues can be avoided. Once shafts and blades are removed from the mixing vessel, the ram press plate is attached and vacuum is pulled, removing troublesome air pockets that may be trapped within the product. A vacuum-equipped ram press expedites the process of removing product from the mixing vessel, eliminates large air pockets in the product (as well as air incorporated in the mixing process), and reduces product waste associated with manual removal of the product from the tank, which in turn reduces the associated cleanup time required for the mixing vessel, as it has essentially been “scraped clean.”
The advantages offered by a change-shaft/change-can-style mixer and vacuum ram press are many, particularly for smaller companies or product divisions. Capital costs—as well as operating, maintenance, labor and product production costs—can be significantly reduced while maintaining or increasing productivity levels and simultaneously ensuring a high standard of product purity.
For more information, contact the author at (323) 560-4723, ext. 216, or sshira@myersmixers.com.
References
1. Westhues, Ulrich, Polyurethanes: Coatings, Adhesives and Sealants, Hannover, Vincentz Network, 2007.
2. Berg, John C., Wettability, New York, M. Dekker, 1993.
wo-part adhesives are an ever-growing segment of the adhesive and sealant industry, with applications in aerospace, construction, automotive, consumer goods, and the DIY/repair sectors. With the U.S. accounting for roughly one-third of the adhesives used worldwide, demand for cost-effective, efficient products that meet the desired criteria—formulating, mixing, metering, and dispensing—are all critical components that must be identified and optimized to ensure that quality and quantity demands are met.
Many different starting materials are available in the formulation of two-part adhesives, all of which will require a variety of mixing, dispensing and application methods. A discussion of such breadth and depth is covered in the wide assortment of texts,1 with this article focusing primarily on two-part (2K) polyurethane (PUR) reactive adhesives, which cure at room temperature, and the mixing conditions
to consider when selecting equipment.
Table 1 (p. 54) provides a brief summary of various properties that should be considered throughout the numerous steps in the manufacture of a 2K PUR system.
Ambient-Cure 2K PUR Adhesives
2K PUR adhesives, which cure at
ambient conditions, are comprised of:
1) polyisocyanates that are either low-molecular-weight or that can be pre-reacted and 2) a low-molecular-weight polyol component. This variety of reactive PUR adhesive is ideal when good heat stability of the joint is required, and is achieved through chemical crosslinking of the polyol and isocyanate through a polyaddition reaction, which forms urethane groups.1
The most visible use for PUR adhesives is two-part systems that have brief working times followed by a curing period, during which the two components crosslink chemically or react with one another to provide the finished material. It is the combining of these two components that produces the physical properties of the finished product and the strength of the bond. The ratio of each component used can be thought of like a recipe; for example, where varying ratios of water, flour, and additives are combined and how they are allowed to “cook” together results in food products as varied as bread, pasta, and cake.
The need for stoichiometric quantities of each component is apparent; the molecules react with one another in specific ratios, and if there is too much or too little of any given component, the quality of the joint will be affected. Isocyanate should be on the order of approximately 10% excess above the stoichiometric ratio required to complete the reaction. The reason for this excess is to ensure adequate crosslinking is achieved even in sub-ideal environments (e.g., residual moisture or environmental impurities on substrate surfaces, inadequate homogenization of the two components, etc.). This ensures that the bond will be strong enough to effectively transfer energy through the complex material and thereby perform its function.
Much effort, energy, money and time goes into formulating a new product—and rightly so, as there is potential for great gains when expectations are met or exceeded. The chemical and physical interplay of components in a formulation is given great consideration, as well as the physical requirements of the mixing and dispersing equipment to execute the goal. However, these are viewed as discrete entities (or areas of project management) that overlap little, if ever. The very nature of the constituents of 2K PURs results in mixing considerations that many will overlook and few—if any—outside of the dispersion equipment industry are aware of such obstacles, or the equipment available to remedy such impedances. When one considers not only the mixing requirements of each component (or the mixing requirement of the two parts combined) but also how the components and parts behave in contact with the mixing equipment, a more complete picture is formed, largely due to interface phenomena.
High-surface-energy materials are
solids with a surface energy greater than 37 dynes/cm (dyn/cm = 10-3 N/m). Liquids “spread” to a greater extent on solids with a high surface energy; that is, the liquid will exhibit a decreased contact angle on a solid of high surface energy vs. a solid of low surface energy. The contact angle can be found through experimentation, or through use of the Young’s equation when the surface energy (or surface tension, for liquids and gases) for all moieties is known.
Pioneering work by William Zisman, Ph.D., in the areas of wetting, surface tension, adhesion and contact angle aided the understanding and interconnectivity of these topics. What we glean from Zisman’s work is: “A surface is more wettable when YLV [the surface tension between the liquid and vapor] is low and when U is low. He termed the intercept of these lines when the cos U = 1 as the critical surface tension YC of that surface. This critical surface tension is an important parameter because it is a characteristic of only the solid.”2
In addition, the majority of molecular liquids achieve complete wetting (U = 0°)
with high-energy surfaces such as stainless steel, which has a surface energy range of 700 to 1100 dyn/cm.
Due to the surface energy, contact angle, and surface (interfacial) tension between the solid (stainless steel), liquid (A or B part of 2K PUR adhesive), and the vapor phase (air, vacuum, or vapor caused by volatilization of the A/B components), the cleanup process between batches is costly. Time that the disperser is not in use for cleaning lowers plant productivity, and due to the high surface energy of stainless steel, it is highly wettable, and thus more difficult to clean (where cleanability is technically defined as the inverse of wettability).
Dispersing 2K PUR Adhesives
For high-quality (very low contamination levels) 2K PURs, even the smallest of contaminations could pose a threat to the finished products’ quality. To ensure that cross-contamination is virtually eliminated, a dispersion system was developed with interlocking removable shafts, bearing housings, tank cover and mixing vessel. This system eliminates batch-to-batch contamination while increasing disperser productivity by reducing downtime between batches. In addition, the system can reduce capital demand by requiring less disperser floor space, which also enables even smaller facilities to produce the desired amount of product.
With reduced potential instances for human error in the dispersion process and a streamlined, simplified start-to-finish process, working costs can also be reduced. Reduced machine cleaning time (mixing vessel, tank lid, shafts, blades, etc.) reduces labor costs while improving the efficiency of each dispersing unit and simultaneously increasing the plant’s productivity. A rugged change-shaft/change-can style disperser was designed for continuous use, with periodic maintenance comprising the majority of a small non-operational fraction of gross potential working time.
Because two-part adhesives are often sold in small individual amounts, one batch each of the A and B component produces many units for purchase by consumers. For this reason, a smaller company or product line may not warrant the purchase of two autonomous mixers—one for the A part and one for the B part. Conversely, using one standard mixer will result in either producing multiple batches of one part of the formulation, or producing in an A-B-A-B sequence, which will have product ready for shipment in less time after production, but will result in significantly more downtime as the machine is thoroughly cleaned between each batch.
As previously discussed, stainless steel—used to manufacture the majority of “clean” surfaces in mixing vessels—has a relatively high surface energy, and liquids tend to spread (i.e., wet) on surfaces with high surface energy. As a result, the contact angle the liquid formed with the stainless steel is reduced when compared to a solid of low surface energy, and is said to be wettable, or have higher wettability than a low-surface-energy solid. Due to the fact that the physical interplay between the adhesive components and the stainless steel promotes spreading wetting on the stainless steel surface, it may be more difficult to fully remove all contamination from the surface. This is explained by the fact that wettability and cleanability are properties of a material that are inversely related through contact angle, surface energy, and the critical surface tension.
Mixing 2K PUR Adhesives
Viscous products that do not flow under their own gravity can present challenges during product discharge and packaging. Traditionally, viscous, non-flowing and/or shear thickening fluids are removed from the mixing vessel with the assistance of a ram press. A basic ram press design consists of a depressible plate, which extrudes the product through a valve at the bottom of the mixing vessel. With a thick product (e.g., one that “holds peaks,” similar to whipped cream), removal of the shafts will create a void in the product. This can result in an entrapped pocket of air, which may be forcefully expelled from the vessel as the ram press advances—a result of the air compressing when pressure is applied and expanding in volume upon approaching the exit valve or contact with the atmosphere. This not only results in potential time delays due to cleanup, but also due to improperly filled product cartridges.
Many options are available to modify a ram press for a specific application; for viscous 2K PURs, a vacuum-assisted ram press minimizes cleanup, product waste and cartridge filling errors. By using a vacuum-equipped ram press, many cartridge filler and product consistency issues can be avoided. Once shafts and blades are removed from the mixing vessel, the ram press plate is attached and vacuum is pulled, removing troublesome air pockets that may be trapped within the product. A vacuum-equipped ram press expedites the process of removing product from the mixing vessel, eliminates large air pockets in the product (as well as air incorporated in the mixing process), and reduces product waste associated with manual removal of the product from the tank, which in turn reduces the associated cleanup time required for the mixing vessel, as it has essentially been “scraped clean.”
The advantages offered by a change-shaft/change-can-style mixer and vacuum ram press are many, particularly for smaller companies or product divisions. Capital costs—as well as operating, maintenance, labor and product production costs—can be significantly reduced while maintaining or increasing productivity levels and simultaneously ensuring a high standard of product purity. ASI
For more information, contact the author at (323) 560-4723, ext. 216, or sshira@myersmixers.com.
References
1. Westhues, Ulrich, Polyurethanes: Coatings, Adhesives and Sealants, Hannover, Vincentz Network, 2007.
2. Berg, John C., Wettability, New York, M. Dekker, 1993.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!