Understanding OSHA's Revised Hazard Communication Standard
Chemical producers and shippers will encounter challenges with the GHS - here's what you need to know.











As a response to the multiple definitions of hazard—and multiple ways of communicating these hazards—the United Nations adopted the Globally Harmonized System for Classification and Labeling of Chemicals (GHS) in 2003. The U.S. Occupational Safety and Health Administration’s (OSHA) revised Hazard Communication Standard has presented manufacturers, formulators and distributors with the challenge of revising their safety data sheets (SDSs) and product labels by June 1. These changes are based on the third revision of the GHS.
The GHS system is gradually being adopted on a worldwide basis. Challenges include the mandatory use of the color red, the potential need for multiple languages if shipping to other countries, various U.S. state issues like New Jersey’s “Right to Know” that go beyond OSHA’s requirements, and many other regional regulatory requirements for compliance in the global marketplace. The reality is that virtually every hazardous chemical product label is subject to change and, in many cases, will require changes on an ongoing basis into the unforeseeable future.
Complicating the environment in which these regulations will go into effect is the fact that the chemicals industry is facing a major challenge due to many large companies’ decentralizing their hazard communication work processes. In addition, many medium- and smaller-sized companies don’t have the internal resources to create their own SDSs and must outsource. Because of the additional requirements in the 2012 OSHA and GHS regulations to be implemented starting June 1, regardless of how or where an SDS is created, automated systems will need to be able to pull the information from Section 2 of the SDS onto labels. In addition, many complicated nuances currently exist in labeling, such as having many different products of various shapes and sizes, the need to respond to customer requirements, the need to access transactional data, languages, and branding information.
Why the GHS?
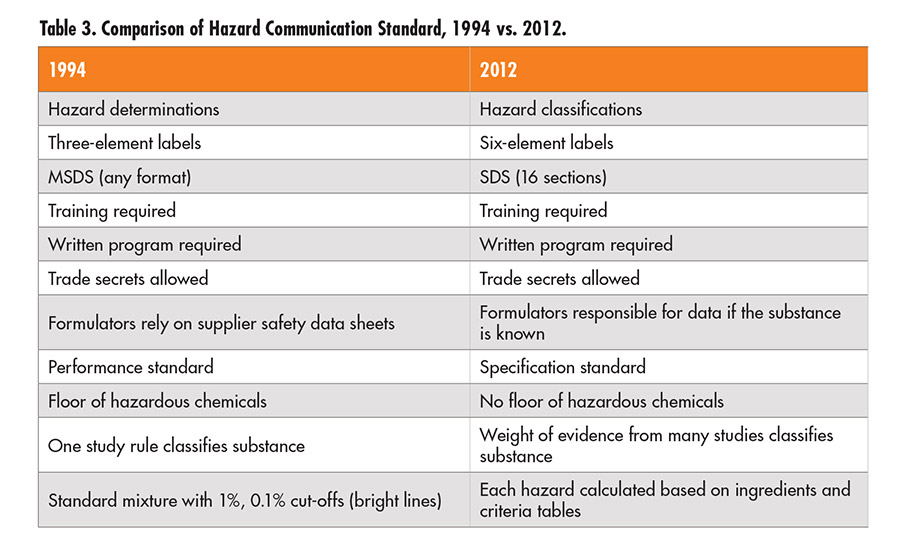
Before adopting the GHS, multiple systems and definitions of hazard were the rule. Even in the U.S. there have been—and, to some extent, still are—different definitions of various physical and health hazards presented by chemical substances. GHS creates a common basis for frequently encountered hazards. The previous definition for flammability, for example, will be replaced by a unified definition (see Table 1).
Timing is an additional concern. For example, the European Union (EU) adopted GHS for substances in 2010, and the classification and labeling of mixtures is scheduled to become mandatory by June 1. Canada is actively working on the institution of GHS but will not be able to complete implementation for industrial products this year. Accordingly, it is trying for mandatory manufacturer implementation by June 1, 2016, and a complete implementation by June 1, 2017, when stock on shelves can no longer be shipped with older formatted labels. Therefore, between June 1, 2015, and June 1, 2016, shippers in the U.S. may need to create a separate label for Canadian shipments.
This difference in implementation timelines is an example of why a single product might need two different labels, depending on its final destination. For the time being, industrial and consumer labels in the U.S. and Canada will continue to differ; by next year, European industrial and consumer labels will follow the same classification and communication scheme.
Oral toxicity is even more complicated than flammability; Table 2 shows the unified definition developed under GHS. There are different GHS tables for dermal toxicity and three for inhalation, one each for gases, vapors, and dusts and mists. All will have to be examined in creating a new label.
In addition, the various shapes of symbols and graphics used for hazard communications are being unified into a single shape and graphic that will be used for both transport and for workplace notification. This will require a change for all EU labels for mixtures—both industrial and consumer—beginning June 1, and will change Canadian industrial labels by June 1, 2016. The new graphic will be mandatory.
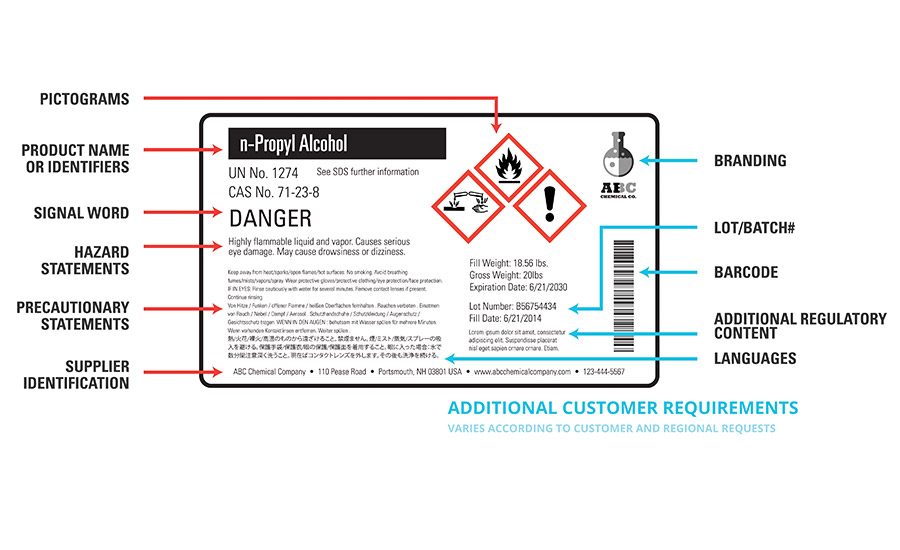
Labels will now contain more information, and will have to be revised to include symbols, standard signal words, and standard phrases (see Figure 1). Other text, such as contact phone numbers and statements about ingredients with unknown toxicity, will also be required. Because of the regional challenges presented by a widening global supply chain, signal words and phrases must be translated into multiple languages, making labels more efficient instruments for global hazard communication.
Before 2015, manufacturers could meet the OSHA requirements as a performance standard by methods of their own choosing. Now, as a specification standard, manufacturers must follow methods of compliance outlined by OSHA. Beginning this year, manufacturers will have to examine all available information and make a scientifically based determination where conflicting toxicity information is found. In addition, formulators will now have a greater degree of responsibility for determining the correct hazards associated with ingredients supplied by others where the identity of the ingredient is known.
Definitions have expanded, especially for physical hazards. OSHA used to talk about flammability, pressure, explosivity and reactivity. Physical hazards are now more finely defined by GHS into the following categories:
• Explosives
• Flammable gases
• Oxidizing gases
• Pressurized gases (compressed gases, liquefied gases, refrigerated liquefied gases and dissolved gases)
• Flammable liquids
• Flammable solids
• Self-reactive substances
• Pyrophoric liquids
• Pyrophoric solids
• Self-heating substances
• Water reactive producing flammable gases (oxidizing liquids)
• Oxidizing solids
• Organic peroxides (corrosive to metals and explosive dusts)
Likewise, health hazards have been more finely defined, but the change is not as dramatic as with physical hazards. The increased number of physical hazards is more in line with existing worldwide definitions for the transport of dangerous goods. The changes to health hazards had to accommodate the various international systems with the guiding principle that no country would reduce the level of protection that previously existed. This will impact both SDSs and labels.
The older definitions of health hazards include irritants, corrosives, toxins, sensitizers, and effects on target organs (e.g., liver, kidney, nervous system, blood, lungs, mucous membranes, reproductive system, skin, eyes, etc.). The newer definitions include:
• Acute toxicity, oral
• Acute toxicity, dermal
• Acute toxicity, inhalation
• Aspiration hazard
• Skin corrosion/irritation
• Eye corrosion/irritation
• Respiratory sensitization
• Skin sensitization
• Germ cell mutagenicity
• Carcinogenicity
• Reproductive toxicity, fertility
• Reproductive toxicity, development
Specific target organ toxicity (single dose and repeat dose)
Most of these categories had previously been regulated; now, all categories are being regulated. It is important to clarify that OSHA will not regulate materials of lower toxicity that would be in the home where children are present. This is because the Consumer Product Safety Commission regulates consumer labels, and that organization has yet to propose adoption of the GHS system.
Where Do You Start?
Manufacturers need to begin by classifying their products. If dealing with pure substances, this will be an easier task than if classifying mixtures. Either way, the criteria from the GHS is the rule. Classification will take longer than the old methods, and organizations need to begin this process now. The challenge is to work with accurate data. GHS does not require “testing,” but it does require obtaining whatever information is available to accurately assess products.
Some items may calculate out to be more toxic, as OSHA has expanded the definition of “toxic” from a level of 500 mg/kg to 2,000 mg/kg in order to be consistent with the GHS. On the other hand, removal of the old 1% bright line means that you have 1% or more of a material with a certain health hazard, and mixtures will not automatically inherit that hazard. For example, some items formerly labeled as irritants may no longer be classified as such. So the classification and sub-classification (known as “categories”) must be dealt with first.
Next, the GHS criteria will lead to the selection of symbols, signal words such as “danger” or “warning,” statements of hazard, and statements of precautions. These all have to go into Section 2 of the 16-section format of the SDS.
Following these considerations is an important issue: since label content will appear on the SDS, both the SDS and the label need to be deployed together. SDSs are documents and can be sent out both in paper or as electronic files, but labels need to be applied to the actual package, which is not as easily accomplished. Key challenges of label production that must be accounted for include accommodating for color printing, dealing with different-sized products, accommodating multiple languages, and transactional data.
It’s common knowledge that OSHA requires a red border on all symbols used to communicate hazard categories. For many companies, using pre-printed labelstock with red diamonds has become less practical, as the number of possible variations of pictograms needed varies and also requires manual oversight to make sure the correct label stock is being used (see Figure 2).
Package size is also an important consideration in labeling, as chemicals can be transported through supply in containers that vary in size from drums to small vials. The label needs to address both OSHA regulations and the size restrictions of the container. However, for small packages, it is a challenge to effectively place a label on such limited space.
Then there is the issue of dealing with languages on a label. In the U.S, English is mandatory; other languages are optional. For most other countries, such as in Europe, the label must be produced in that country’s language but may also require other languages if the item is sold or transported in other countries.
Extending the challenge of GHS labeling is a common requirement to apply transactional data such as batch numbers, lot numbers or packing dates. This data, in conjunction with the variables of color, size and language, introduce complexity on the label that make pre-printing labels impractical.
Real-time, data-driven labeling is one of the primary pathways of dealing with these issues to ensure that the correct symbols, languages, and transactional data appear on labels of any size or shape. This approach also enables manufacturers to leverage the same regulatory content to ensure that the SDS and label agree with each other. The last thing you want is for the SDS to say one thing, and the label to say something else.
Ongoing regulatory changes in the chemical industry, successful GHS compliance, and regional regulatory adherence all require rapid labeling changes to be deployed quickly throughout the organization. The ultimate goal is meeting the requirements presented by the GHS while also dealing with the complexity of labeling hazardous materials to protect all participants in the global supply chain. To achieve this goal, companies must first understand the impact and changes that the GHS necessitates while pursuing an approach that accounts for the unprecedented level of complexity and change required for labeling in the chemical industry.
For more information, visit www.loftware.com/ghs.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!