A Safer Alternative Takes the Pressure out of Formulating Pressure-Sensitive Adhesives
A new, tetrafunctional epoxy reactive modifier is increasingly being used as non-mutagenic, drop-in formulation replacement for polyfunctional aziridine.
















Formulating pressure-sensitive adhesives (PSAs) presents a number of complex challenges. The adhesive must flow and set without activation from anything more than the pressure of a hand, remain permanently tacky, and exhibit a strong bond—often to a variety of substrates, such as wood, glass, or metal—for as long as the application requires or until the end user decides to remove it. Once removed, the PSA must exhibit the necessary cohesive strength that it can be easily removed without leaving adhesive residue behind.
Crosslinkers are a critical part of the two-component package used to create acrylic, water-based PSAs (the first part being the monomer, to be discussed later). The wide span of tapes, labels, protective films and more that use this technology rely on an effective crosslinker to achieve many of their key performance properties, including cohesion, shear strength, chemical resistance, performance at high or low temperatures, and peel adhesion. Essentially, it is the crosslinker’s function to ensure the ultimate performance success of the adhesive; without it, the PSA would fail to meet the criteria required to satisfy its intended purpose.
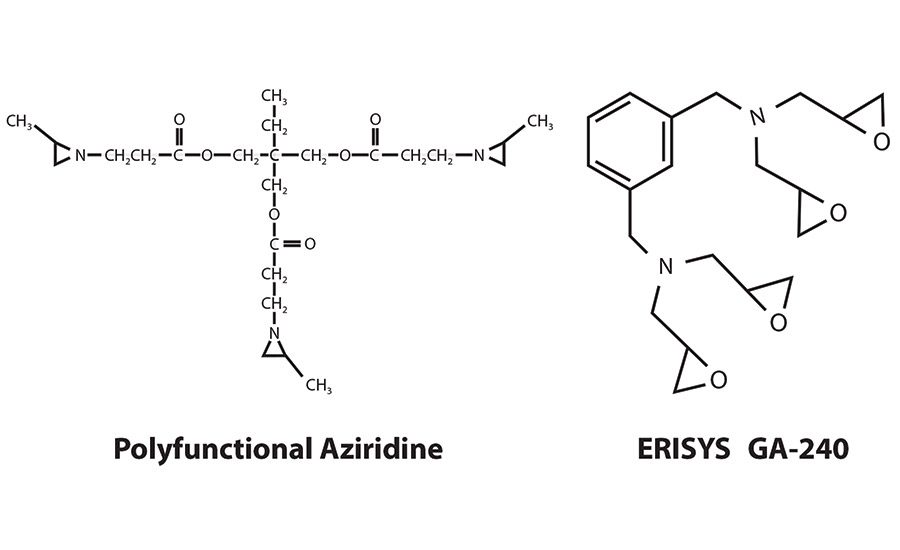
Polyfunctional aziridine is a common choice as a crosslinker in many waterborne PSAs due to its efficacy and economy. However, polyfunctional aziridine has recently come under fire in both the U.S. and EU markets due to its status as a potential mutagen. Its reduced efficacy over relatively short dwell times in water is a result of decomposition due to hydrolysis; the net effect can lead to variation in adhesive properties, as well as leading to problems with manufacturing efficiency. These challenges have added to the concerns that a PSA formulator must consider and led to a growing need for a crosslinker option that is less hazardous to human health, more stable over time and can also offer the same or greater hardness and shear adhesion for adhesive formulations. One alternative* is a new tetra-glycidyl m-xylene diamine, a tetra-functional epoxy reactive modifier, that has been shown to be an effective replacement for polyfunctional aziridine crosslinkers.
Formulation
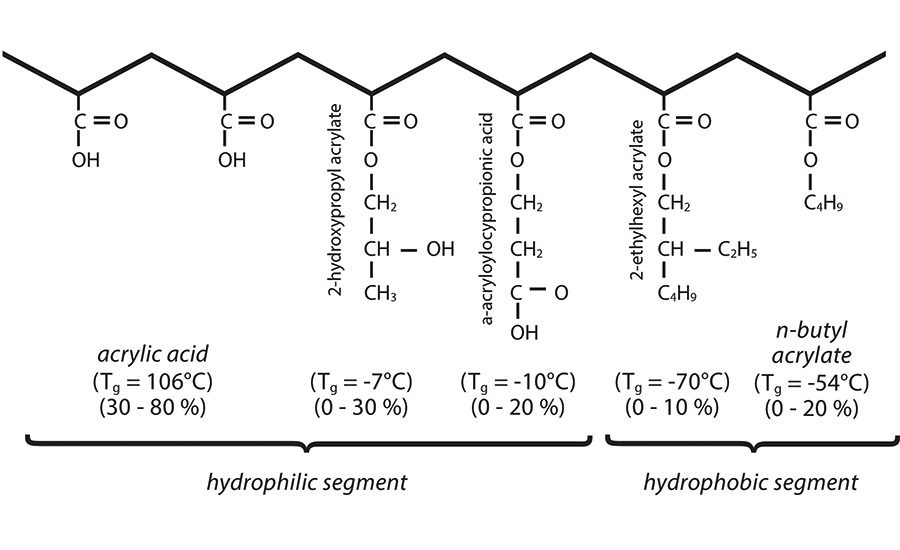
Before crosslinking comes into consideration, formulators must first select the correct monomer. Monomers, which are used to produce polymeric substances, must be carefully chosen in order to control the ultimate physical properties of PSAs. Hydroxyl and acid functional monomers help to make the polymers hydrophilic, thereby increasing water solubility, and also function to help increase adhesion. Monomers containing longer alkyl chains help to decrease the glass-transition temperature (Tg) of the polymer, thereby increasing the tack and peel adhesion and decreasing shear adhesion of the adhesive.
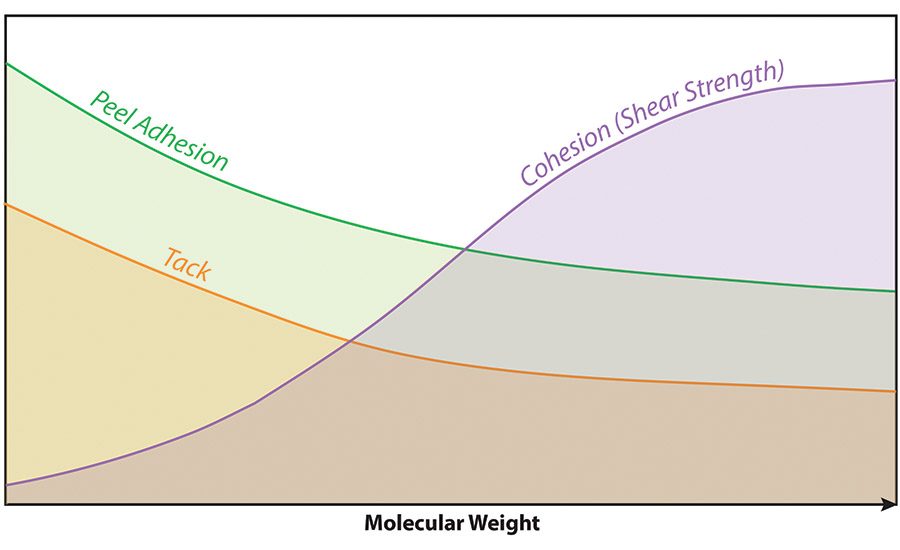
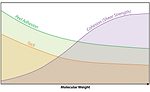
Some of the more common monomers used in making acrylic polymers for PSAs are shown in Figure 1, along with their typical use levels and Tg values. Molecular weight is of prime importance, as it will have an impact on cohesive strength, peel adhesion and tack, as shown in Figure 2. It should be noted that HMW polymers are also typically more difficult to process.
Monomers also have another important function in a PSA formulation: providing sites for crosslinking to occur. The functionalized acrylic resins in many PSAs require the use of a crosslinker in order to control tack and adhesion. The crosslinker also performs the essential function of preventing cold flow, as the polymers used in these adhesives normally operate at temperatures above their glass transitions and would otherwise tend to cold flow at relatively low temperatures.
Typically, the crosslinker is added to the PSA just prior to coating the substrates, and then heat is applied to drive off water during processing of the coated films to help activate the interparticle crosslinking reaction. The result of the reaction—during which links are formed between polymer chains within neighboring particles—is increased molecular weight of the polymer, as well as improved cohesion, higher shear strength, lower peel adhesion, less tack, higher temperature performance and improved chemical resistance.
While polyfunctional aziridine is a widely used crosslinker for acrylic PSA applications, it has recently been associated with a number of health, safety and environmental (HS&E) challenges that have created some barriers for its use. In addition to being classified as corrosive/irritating to the eyes and skin and being linked to the development of occupational asthma (ACD), it is suspected of causing genetic defects, labeled as a GHS category 2 germ cell mutagen.
The new diamine is a tetrafunctional epoxy modifier based on meta-xylenediaminea nitrogen-containing, highly reactive resin with a very low viscosity that is being increasingly used as a safer, non-mutagenic, drop-in formulation replacement for polyfunctional aziridine. As well as being a non-mutagen, an additional benefit is its ability to remain effective in a formulation over time. In contrast, polyfunctional aziridine decomposes over a matter of days, causing the need for the formulator to check the spend of the leftover crosslinker and re-dose the system by adding more crosslinker to account for the difference.
Testing and Results
Two crucial performance criteria to determine the success of a waterborne PSA were selected in order to evaluate the efficacy of the diamine epoxy reactive modifier relative to a common polyfunctional aziridine crosslinker: peel adhesion and shear adhesion (cohesive strength).
Tests were run using a tetrafunctional glycidyl amine and a polyfunctional aziridine as crosslinkers for acrylic emulsion for PSAs. For peel adhesion testing, crosslinkers at levels of 1-5% (dry basis) were added to the acrylic emulsion under agitation. For shear testing, levels of 0.25-3% (dry) were used. The poly- functional aziridine was added in the form of a 65% solution in ethyl acetate. The tetrafunctional glycidyl amine was added “as is.” Draw-downs were made on polyester film using a #50 wire wound rod. Films were oven dried for 5 min at 150°F, then held at room temperature (RT) and tested for peel adhesion initially and periodically thereafter up to 35 days later. Shear adhesion was tested after one, three and five days at RT.
Peel Adhesion
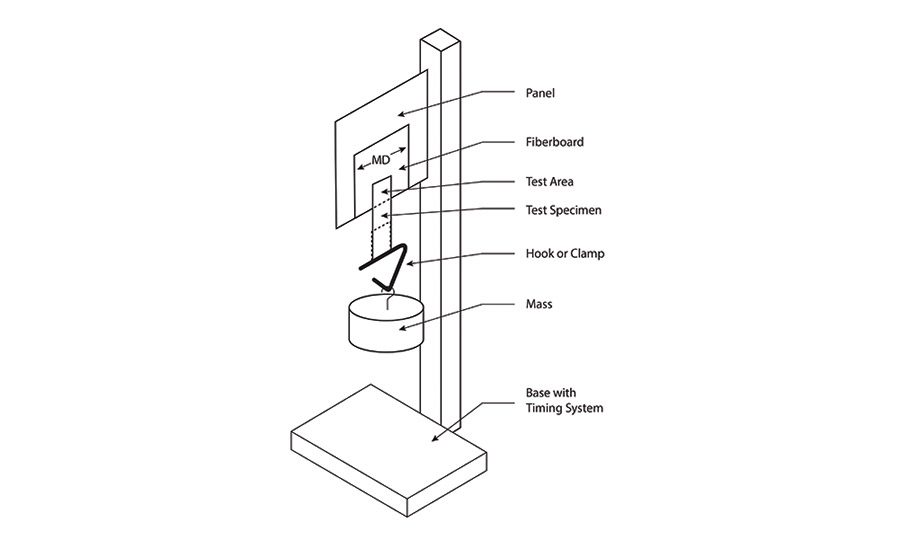
Peel adhesion is normally measured by adhering a 1-in.-wide tape sample to a piece of polished stainless steel, then measuring the force required to pull the tape off using an Instron machine at a controlled rate of speed. The tape is pulled at an angle of 180°, and force requirements from a few ounces to a few pounds are typical. Values are reported in units of “pli” or pounds per linear inch, meaning per 1 in. of width (international units are Newtons per 10 mm of width). Figure 4 (p. 22) shows a typical configuration for a peel test.
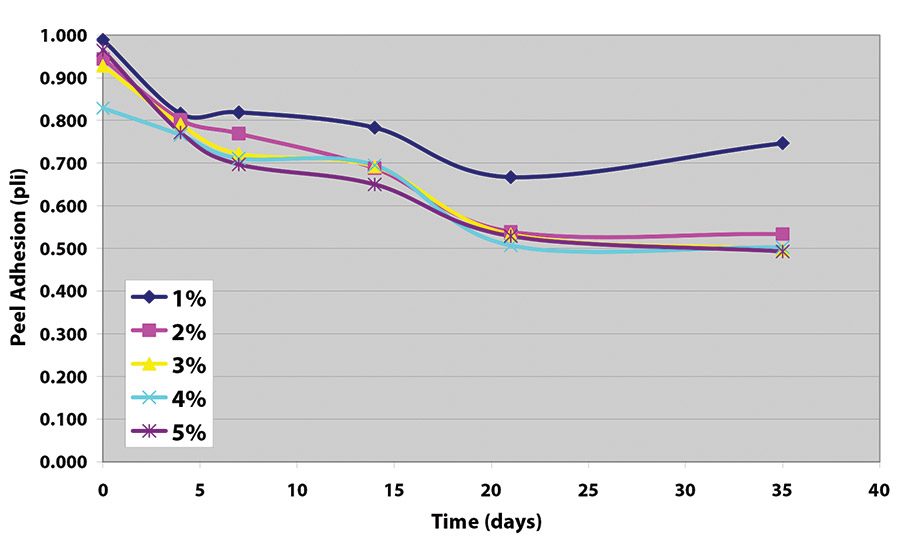
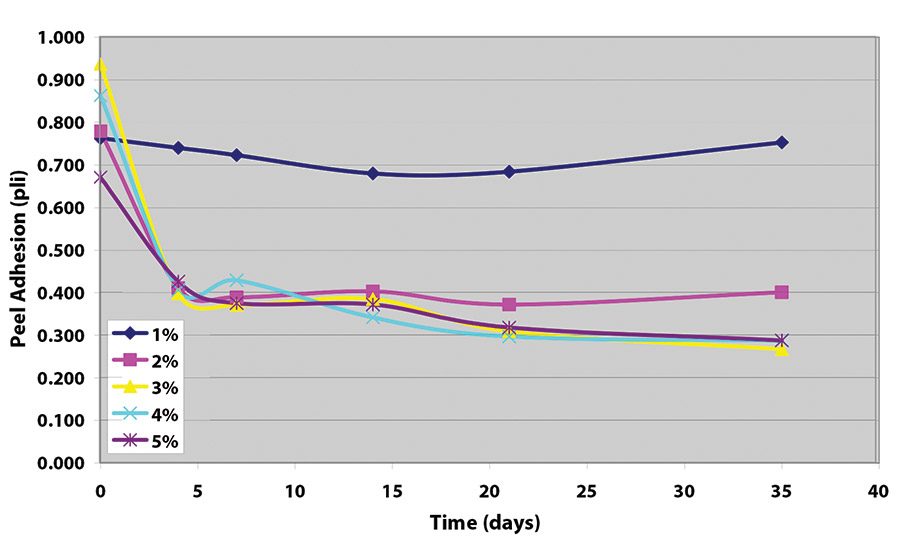
The peel adhesion when no cross-linker was used was measured at approximately 1.5 pli (2.63 N/10 mm). Samples made without crosslinker all show adhesive transfer to the stainless steel adherend.
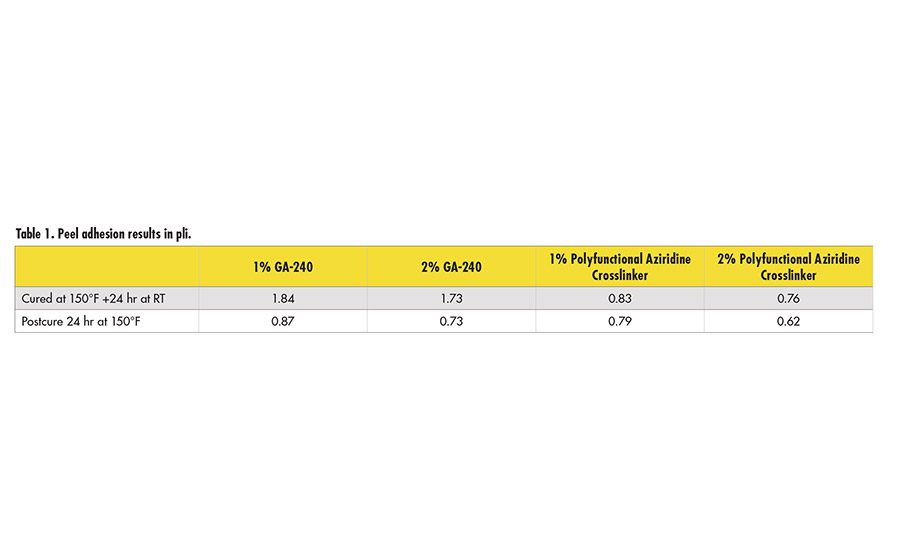
The results indicate that, while each crosslinker works well to decrease peel adhesion, values achieved with the tetrafunctional glycidyl amine are somewhat lower (see Figure 5 and Figure 6). Further work indicates that equivalent levels of adhesion can be achieved with somewhat higher levels of the epoxy reactive modifier, as long as the adhesive film receives a force cure (see Table 1).
Shear Adhesion
In contrast to peel adhesion, shear adhesion is a static test where a 0.25 in.2 (½-in. x ½-in. or 12.5 mm x 12.5 mm) piece of tape is adhered to a stainless steel plate. A typical setup is shown in Figure 7. A 500-g weight is hung from the tape, and the time it takes for that piece of tape to pull off the substrate is measured.
Shear adhesion is an important indicator of cohesive strength, which is important in order to prevent adhesive transfer to the substrate when removing the film, tape or label. Figure 8 illustrates the difference in adhesive with poor cohesive strength (on the left) vs. good cohesive strength (on the right). The longer time to failure in the shear adhesion test is indicative of greater cohesive strength. Most PSAs, even with a small amount of crosslinker, show times to failure in excess of 3,100 min (with a 500-g weight).
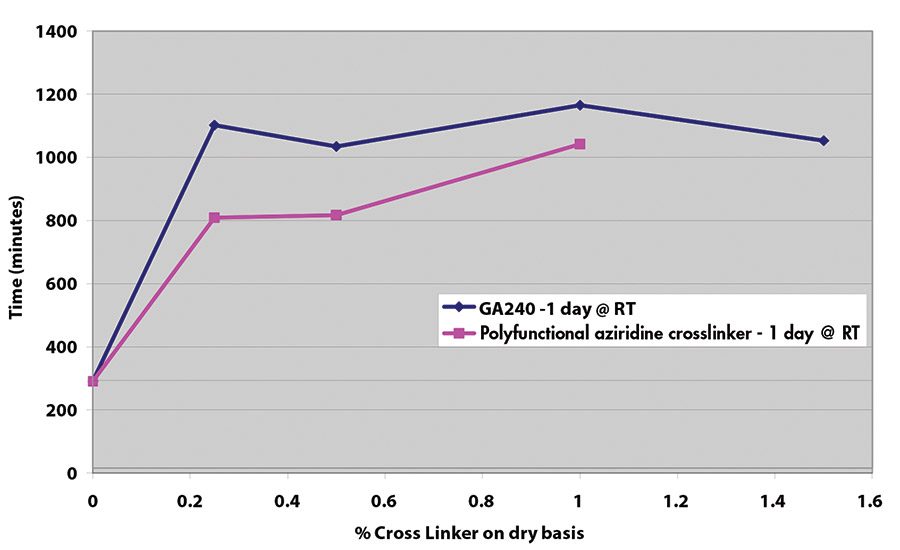
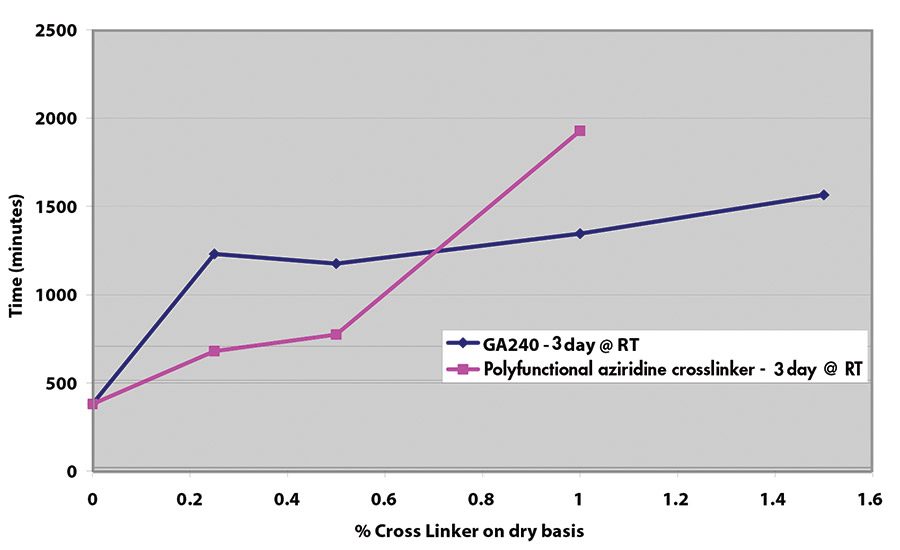
Results for shear adhesion testing indicate that the epoxy reactive modifier is more effective than another crosslinker at lower use levels (see Figures 9-11). It is important to understand that at crosslinker levels in excess of those shown on graphs, the shear adhesion values were in excess of 3,100 min.
Conclusion
Results of testing for key performance properties for PSAs illustrate that the new epoxy reactive modifier, a tetrafunctional glycidyl diamine, is a highly effective and efficient crosslinker in waterborne, acrylic formulations to decrease peel adhesion, increase shear strength and eliminate any problems with adhesive transfer to substrates by increasing the cohesive strength of the treated adhesives. The epoxy reactive modifier demonstrates equivalent performance to the polyfunctional aziridine crosslinker tested in terms of shear adhesion and increased cohesive strength. While the decrease in peel adhesion with the epoxy reactive modifier is not as extensive as with the aziridine crosslinker tested, the epoxy reactive modifier exhibits a good balance of performance properties and does create substantial and entirely acceptable reductions in peel adhesion in PSAs.
In addition to enhancing performance of PSAs, the epoxy reactive modifier also offers benefits that the polyfunctional aziridine crosslinker does not, such as manufacturing efficiency and a safer HS&E profile, making it an attractive alternative. For example, while the tetrafunctional glycidyl diamine will remain active in formulated adhesives for several days, systems treated with other crosslinkers have a short pot life and will require re-inoculation of the adhesive due to decomposition of the aziridine due to hydrolysis. In addition, the tetrafunctional glycidyl diamine does not carry with it the types of safety and health concerns that are inherent in the aziridine systems. As a result, it is a safer, more efficient choice for adhesives formulators looking to replace potentially mutagenic polyfunctional aziridine crosslinkers with an easy drop-in replacement.
Acknowledgement
The author would like to thank Charles Zarnitz for original research on this project.
For more information, visit www.cvc.emeraldmaterials.com.
References
1. Ash, M. (2004). Handbook of Green Chemicals (p. 631). Synapse Information Resources.
2. Kanerva, L., Keskinen, H., Autio, P., Estlander, T., Tuppurainen, M. And Jolanki, R. (1995), Occupational respiratory and skin sensitization caused by polyfunctional aziridine hardener. Clinical & Experimental Allergy, 25(5): 432–439. doi:10.1111/j.1365-2222.1995.tb01074.x
* ERISYS® GA-240
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!


















