A Natural Choice for Adhesives and Sealants
Cashew nutshell liquid-based epoxy curing agents and polyols offer outstanding performances and benefits for adhesives and sealants.









Cashew nutshell liquid (CNSL), a bio-renewable resource found in the honeycomb structure of the cashew nutshell, is considered a byproduct of the cashew industry. Therefore, CNSL is a non-food chain product that would otherwise be disposed of. Cashew trees can grow abundantly in tropical conditions.
One of the most commercially useful chemicals from CNSL is cardanol, a pentadecadienyl phenol with an aliphatic side chain that usually consists of a mixture of one, two, and three double bonds in a linear chain. This natural phenolic compound has interesting chemical structural features that enable a variety of chemical modifications to create bio-based monomers and polymers. Phenalkamine is one of most successful chemicals used in cashew nutshell liquid, along with phenalkamide, exhibiting good reactivity for fast and low-temperature cure and strong adhesion even to damp or poorly prepared surfaces. In addition, its long aliphatic side chain delivers excellent water resistance, corrosion protection and chemical resistance.
Materials and Methods
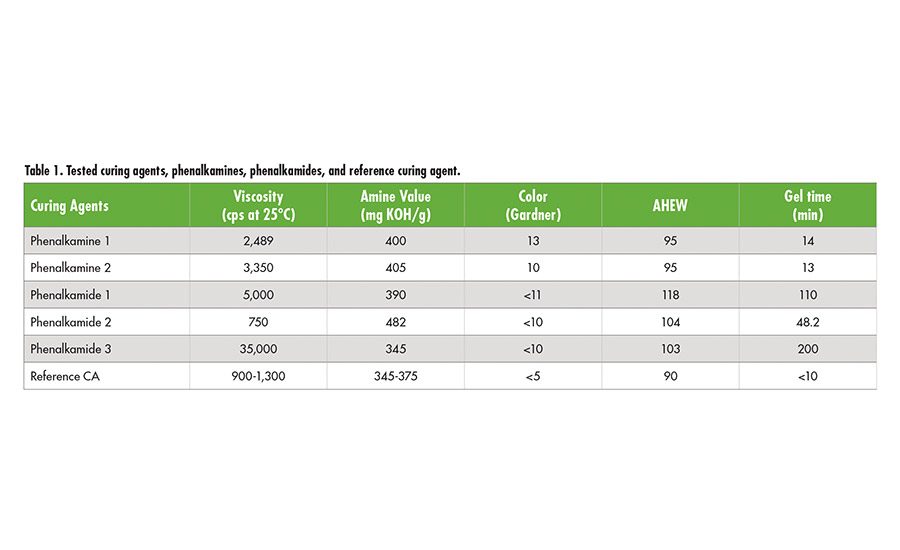
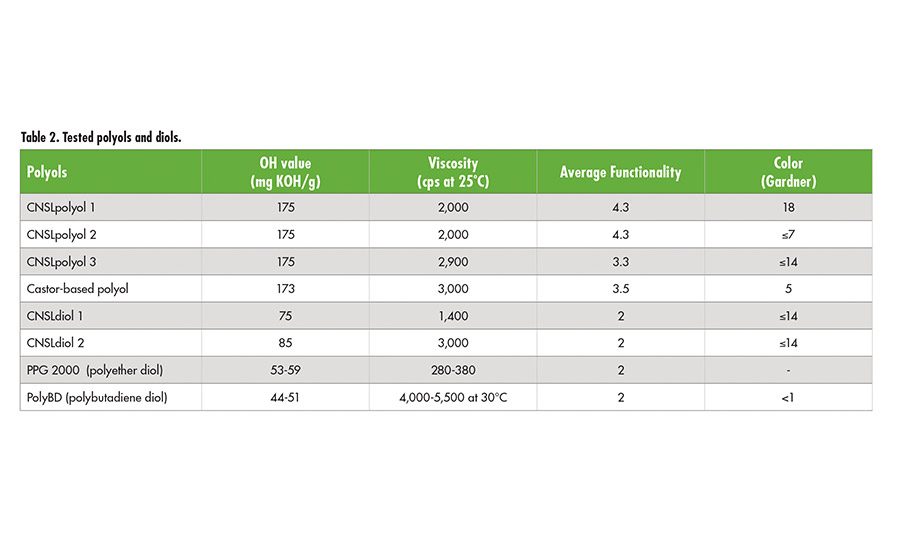
Tables 1 and 2 (p. 22) contain basic information on tested CNSL-based products and reference curing agent, polyols, and diols. The reference curing agent is a Mannich-based product used in civil engineering for fast cure and water and chemical resistance. Phenalkamines and phenalkamides were cured with liquid epoxy (EEW=190) without any additional formulations to determine its mechanical properties, bond strength, and durability. In the case of CNSL polyols and diols, polymeric MDI was used to make polyurethane specimen for its performance.
Test specimens and results were obtained based on the following ASTM methods: lap shear strength (ASTM D1002) on sand-blasted steel, tensile strength/elongation (ASTM D638), flexural strength (ASTM D790), compression strength (ASTM D695)and chemical resistance (ASTM D6943), and Shore D/A hardness (ASTM D2240). Gel time was measured in 50 g amounts at 25˚C by using a gel time meter.
Moisture sensitivity of polyols was determined by measuring the amount of CO2 generated after pouring pre-mixed polyurethane into water. Such blends were kept for one day. It is known that isocyanate can react with water to generate CO2 gas, and greater hydrophobicity less CO2 evolution.
Results and Discussion
Phenalkamines
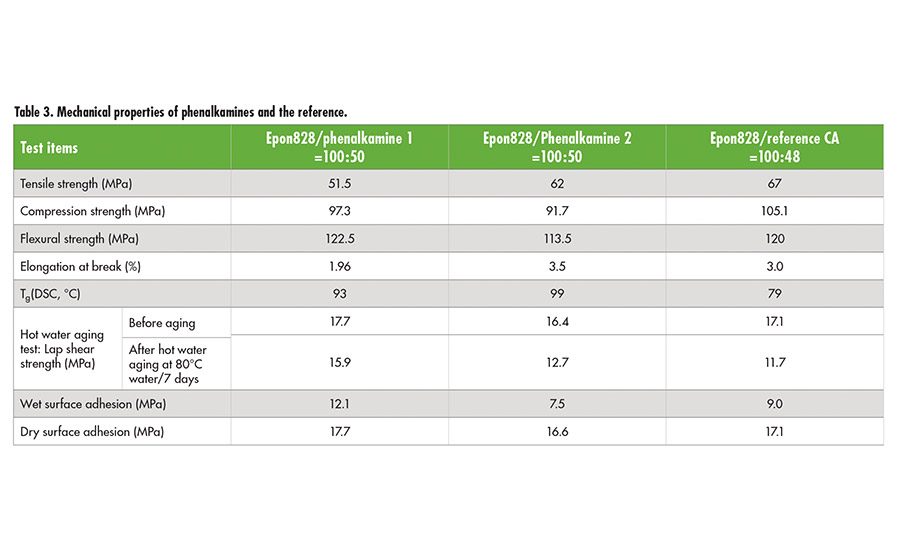
High-performance phenalkamines have been developed that have favorable labeling, faster cure at room and low temperatures (i.e., 0°C), and outstanding mechanical properties. Fast bond strength development at low temperatures of 0-10°C makes phenalkamine 1 and 2 suitable for year-round construction and building adhesives such as grouts, concrete patching compounds, rapid set repair, and maintenance adhesives. These products also offer epoxy construction and building adhesives with the excellent mechanical strength and high Tg required to withstand external stresses during service.
Adhesive materials are exposed to moisture at all times. Absorption of moisture by adhesives can weaken their strength and ultimately result in failure of the material. The phenalkamines provide outstanding moisture resistance and surface tolerance for wet substrates, which is an inherent benefit of CNSL technology that ensures structural bonds stay strong throughout the lifetime of the adhesive. Table 3 demonstrates detailed performance comparisons between the new phenalkamines and the reference. Compared to the Mannich-based reference, phenalkamine 1 demonstrated the best durability, hot water aging resistance, and wet surface tolerance, while phenalkamine 2 provided the fastest strength development at 0°C cure.
Immersion tests for 30 days at room temperature were conducted to determine chemical resistance of the phenalkamines. Tested chemicals include xylene, MEK, gasoline, acid and base aqueous solutions, and alcohols. Both phenalkamines exhibited excellent corrosion resistance and adhesion against most of chemicals except MEK for phenalkamine 1 and 3% NaCl solution for phenalkamine 2. It is also determined that both of phenalkamines can resist up to 175°C without loss of adhesion.
It has been known that the addition
of free phenols or benzyl alcohol can enable amine curing agents to be cured fast at low temperature. However, because of the increasing toxicity concerns about free phenols and benzyl alcohol, safer alternatives with comparable performances have been developed. The newly developed phenalkamines have demonstrated fast strength development at low temperature, excellent mechanical strengths, high Tg, hot water aging resistance, and surface tolerance (see Table 3). In addition, they do not contain any free phenol or benzyl alcohol. According to the MSDS of the reference curing agent, it contains free p-tert-butyl phenol.
Phenalkamides
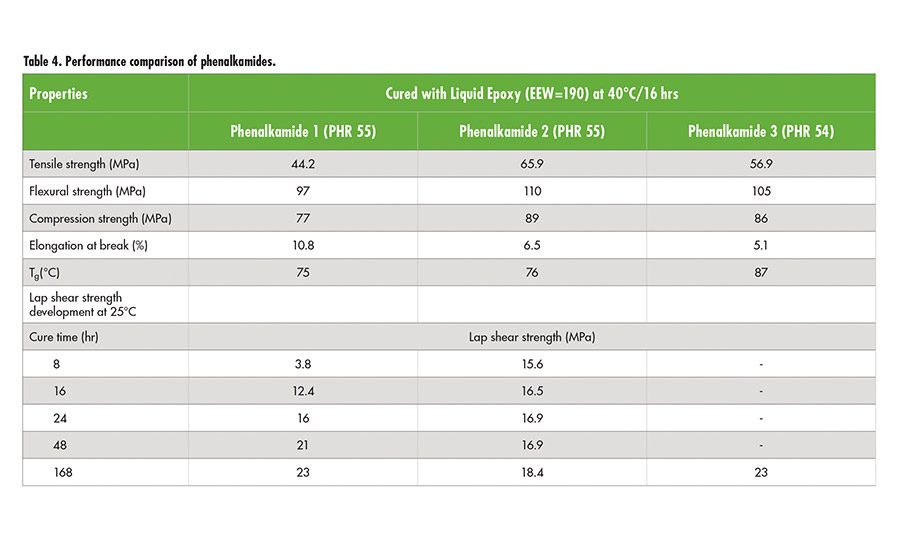
Phenalkamides have been developed to improve the color stability of phenalkamines by chemically incorporating polyamide functionality to cashew-based amines. Previous papers1 have demonstrated phenalkamide’s outstanding performance for coatings, but its applications have been limited due to high viscosity and solvents. Thelatest technology allows the development of light-colored, low-viscosity, solvent-free (no benzyl alcohol), and non-toxic labeling of phenalkamides 1 and 2. These phenalkamides exhibited excellent adhesion strength to various substrates, fast strength developments, overall outstanding mechanical properties, excellent corrosion resistance (2,000 hr salt spray), thermal resistance, and chemical resistance for use in structural epoxy adhesives.
As shown in Table 4, phenalkamide 2 delivers fast bond-strength development combined with excellent mechanical properties such as high tensile, flexural, and compressive strength. Adhesives based on phenalkamide 2 can be used on various substrates, including poorly prepared surfaces as encountered in many construction job sites. Phenalkamides 1 and 3 give longer pot-life and outstanding bond strengths to various substrates. Phenalkamides 1 and 2 are solvent free and low viscosity; therefore, they offer excellent formulation latitude, while solvent-free phenalkamide 3 can provide higher Tg with extended pot life.
Key benefits from phenalkamides compared to phenalkamines include longer potlife, increased bond strength and flexibility;phenalkamines are best for fast and low-temperature cure. Both of the epoxy curing agents, phenalkamines and phenalkamides, have demonstrated outstanding chemical resistance, thermal resistance up to 175°C, and good bond strength to various substrates, exhibiting cohesive failure, such as carbon fiber reinforced epoxy composite, ABS, PC, PVC, and metals.
CNSL Polyols
Polyols derived from CNSL bring unique characteristics compared to widely known polyester and polyether polyols, and other natural based-polyols to adhesive applications. Outstanding hydrophobicity helps to overcome moisture sensitivity of the polyurethane system and ultimately extends service life of the adhesives by preventing moisture absorption and failure from hydrolysis. Polyurethanes use isocyanates that are moisture-sensitive and the absorbed moisture can induce an unwanted side reaction like generation of CO2 bubbles. Polyols with excellent hydrophobicity will reduce moisture sensitivity during blending process of isocynates and polyols, and consequently result in less CO2 evolution. CNSL polyols showed significantly lower CO2 bubbles compared to castor oil based polyol when 100 g of polyol and MDI mixed with 100 g of water.
Chemical immersion tests for 30 days in 10% H2SO4, 10% NaOH, and 30% NaCl were also performed using pigmented systems. CNSL polyols exhibited excellent acid, alkaline and salt resistance with no blisters under all three conditions, while the castor oil-based polyol system showed heavy blister formation in the 10% NaOH immersion test within 6 days of exposure. Excellent chemical resistance of CNSL polyols results from aromaticity and a long aliphatic chain of CNSL molecules. High alkaline resistance of CNSL polyols allows this ingredient to be used in polyurethane concrete adhesives and sealants.
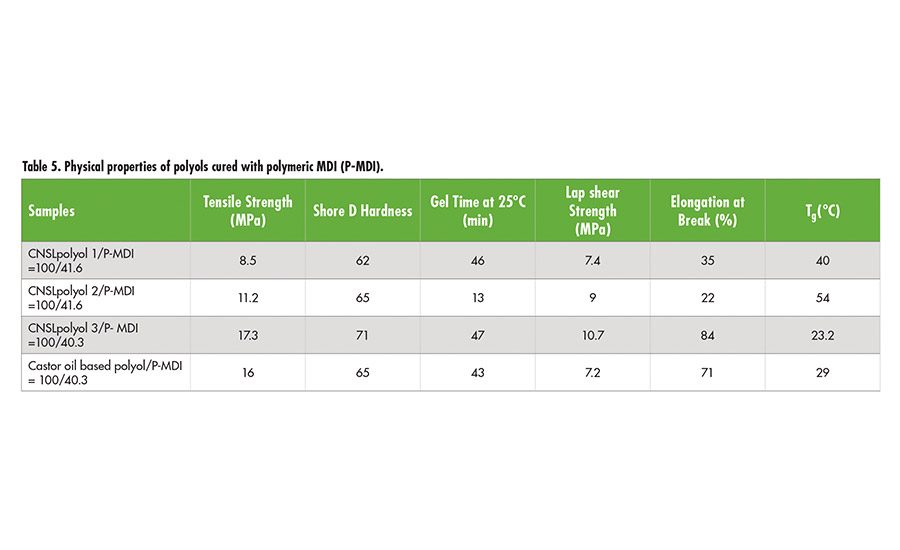
Table 5 contains physical properties of polyols when they are cured with polymeric MDI(P-MDI). CNSLpolyol 2 is light in color and a fast cure polyol compared to CNSLpolyol 1, while CNSLpolyol 3 offers higher tensile strength and elongation with a comparable performance to castor oil-based polyol.
CNSL Diols
CNSL diols can be formulated into flexible foams, elastomers, adhesives, and sealants as a binder or used as a building block to produce polyurethane pre-polymers. Pre-polymers are extensively used in one-component polyurethane adhesives and sealants. CNSLdiols 1 and 2 contain polyester structure on top of high content of CNSL back-born, and their functionality is approximately 2. Because of the chemical structures of CNSL diols, they show increased hydrophobicity, better compatibility and reactivity compared to polypropylene glycol (PPG) diols and polybutadiene diol (polyBD).
Table 6 displays compatibility of CNSL diols with PPG diols, polyBD, ethylene vinyl acetate (EVA), and two different tackifiers (tackifier 1 is aliphatic hydrocarbon resin and tackifier 2 is polybutane-based). CNSLdiol 2 exhibited excellent compatibility to PPG, polyBD, EVA, and tackifier 1, while polyBD has shown limited compatibility to PPG diols. EVA and tackifers are commonly used components in one-component polyurethane adhesives, especially on hot-melt adhesives. CNSL diols also have shown much faster cure speed compared to PPG diols when they are cured with HDI, IPDI and MDI. In addition, compared to polyBD, CNSL diols have cured faster with HDI, but exhibited comparable speed with MDI. It is suggested that the key contributor of the higher reactivity of CNSL diols is increased number of primary hydroxyl.
CO2 evolution was studied to determine moisture sensitivity of diols and isocyanates. The hydrophobic structure of CNSL diols can reduce moisture sensitivity of the polyurethane system, thus minimize CO2 generation when isocyanate react with water. CO2 bubbles trapped in adhesives are considered defects. Minimization of defects will help to improve durability of adhesives.
As expected, CNSL diols outperformed PPG diols but slightly inferior outcome against polyBD. The main use of diols is to make prepolymers that can be used in one-component polyurethane systems. These prepolymers contain isocyanate(NCO) end group, and the percentage of NCO content can vary with performance and process requirements. For this study, 7% NCO pre-polymers were prepared and cured with 1,4-BDO extender in the presence of Dabco 8154 catalyst and BYK054 air release. Pre-polymers made out of PPG diols and polyBD are also studied under the same conditions. Cured specimens were exposed to three different solutions (water, 10% NaOH and 30% H2SO4) for 21 days prior to obtaining Shore D hardness data. CNSL diols and polyBD were found to be much better at hydrolytic stability than PPG diols; this should be due to their superb hydrophobicity.
An additional hydrolytic stability study was carried out under 50% H2SO4 solution. For this study, specimen were prepared through single diol or blend of diols reacting with polymeric MDI at NCO index 100 and cured for 4 hrs/70°C
and 1 hr/120°C. The test results confirmed that the greater hydrophobicity can produce the better hydrolytic stability of polyurethane adhesives. The good compatibility feature of CNSL diols also provided an opportunity to blend with other diols and evaluate their hydrolytic stability. CNSL diols and polyBD blends showed significantly better performance than CNSL diols and polyBD by themselves. Future work can be dedicated to find causes of the synergistic effect using CNSL diols with polyBD.
Conclusion
CNSL-derived phenalkamines, phenalkamides, polyols and diols demonstrated outstanding performance in epoxy and polyurethane adhesives, respectively. The excellent mechanical properties, bond strength, and hydrophobicity of CNSL-based products will allow improved durability of adhesives and sealants during their service time. In addition, CNSL-based products are renewable, solvent free and labelingfriendly. Their high bio-content, coupled with high performance, consistent quality, and globally available supply, make CNSL-based epoxy curing agents and polyols a natural choice in sustainable epoxy and polyurethane formulations. ASI
For more information, visit www.cardolite.com.
Acknowledgments
This study was carried out by the Research and Development Group and Technical Service Team at Cardolite Corp. We thank James Zhao and William Wei for all the technical support. Special thanks to Pietro Campaner from AEP Italy.
Reference
1. Y. Kim, F. Tavares, D. Lawson, M. Chen, J. Dai, American Coatings Conference paper (2012); European Coatings Show Conference paper (2013).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!












