Selecting the Optimal Adhesive for Wearable Medical Devices
Skin-interfacing adhesives’ ability to properly adhere to the skin is a key factor for an effectively functioning wearable device.

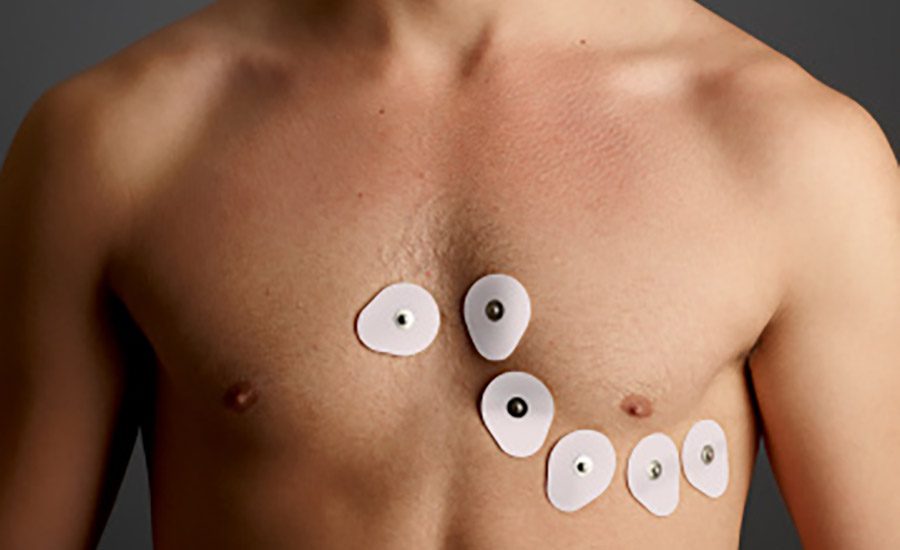


The wearable adhesives market is being propelled by healthcare advancements and increasing demand for personalized care options. Wearable adhesives are used to attach medical devices to the body for continuous and long-term durations. While the skin-interfacing adhesive is only a small component of a wearable device, its ability to properly adhere to the skin is a key factor for an effectively functioning device. The selection and development of this adhesive is challenging and needs careful consideration due to the complex and varying nature of human skin and the characteristics of the specific wearable device, such as its size, weight, flexibility, and application purpose.
Adhesive Requirements
The requirements for an adhesive are stringent; it must be capable of adhering well to dry, perspiring, or sensitive human skin and remain securely affixed even during showering and physical activity, yet be removable without leaving residue on the skin surface and without causing skin damage. In addition, the adhesive should not irritate the skin.
On top of those requirements, skin is a living substrate, which varies among individuals. The main skin properties that affect the adhesion are transepidermal water loss, hydration level, lipid secretion and surface tension. All of these properties vary with the season, the individuals skin and the area on the body the device is adhered to. Therefore, no single adhesive solution meets all wearable adhesive needs.
In order to identify the adhesive that is the best choice for a particular wearable device, it is essential to first define the requirements for the device and its adhesive, including:
• Targeted users
• Application site
• Wear duration for individual application and number of expected re-applications
• Condition of use (at rest or active patient)
• Size and weight of the device
• How the device will be attached to the adhesive patch
• Whether the device will cause some occlusion of the patch or allow sweat to pass though the patch
Prototype Screening
While the most effective way to test a wearable device is to conduct a wear test with healthy panelists, this can be cumbersome, time consuming and expensive. In addition, only a restricted number of prototypes are allowed to be tested through one protocol. To save time during development, the screening process of the possible solutions is fundamental. An effective combination of a few in vitro and in vivo tests can drastically accelerate the development cycle.
Base characteristics of the adhesive are moisture vapor transmission rate (MVTR), peel, tack and shear. All of those properties can be effectively screened in vitro. Comparing adhesive options to one another in terms of tack, peel and MVTR will drive informed decisions on the adhesive selection and will facilitate a new selection in case the first choice of adhesive does not perform as expected in a healthy volunteer study.
Rheology
Rheology is a useful tool to position each adhesive in relation to one another in terms of tack, peel and shear. Tack, peel and shear are a response to the bulk adhesive, not inherent properties of the pressure-sensitive adhesive (PSA) itself.
A correlation between the degree of adhesion and the viscoelastic properties of the adhesive is well known. The viscoelastic nature of the PSA enables it to exhibit both solid and liquid-like behavior. This behavior is described by the elastic modulus (G’), the viscous modulus (G’’) and the creep compliance (J; also called cold flow). Tack of bonding is a low-rate process where the adhesive should be liquid-like and hence flow sufficiently to promote intimate contact with the skin. Peel is a high-rate process where the adhesive should be solid-like (cohesive and internally strong).
Based on this theory, Chu and Dahlquist have developed some criteria for proper adhesion based on G’ and G’’ at low frequency (0.01 rad/s) to represent the tack and at high frequency (100 rad/s) to represent the peel. The first step of development should be to select a number of adhesive options and conduct the rheology test—ideally at skin temperature 32°C—at low and high frequency so that all of the adhesive prototypes can be positioned in comparison to one another, excluding the carrier and the coat weight.1
Skin Surrogates
Alternatively, a skin surrogate may be used. Some of the known substrates are VITRO-SKIN® from IMC Inc. or a skin surrogate such as the one described by Haber.2 VITRO-SKIN, which was initially developed to test cosmetic products, has been found useful to test skin PSAs. The only restriction with those surrogates is the need to set up a standardized preparation technique and use adhesives coated at a similar coat weight on a stiff carrier so that a true evaluation can be made.
Moisture Vapor Transmission Rate (MVTR)
The MVTR of the adhesive selected should be as high as possible in order to avoid de-bonding of the device from the skin due to human sweat.
Biocompatibility Testing
The next step is to generate the proper biocompatibility test on the adhesive prototypes so that it can be safely used on healthy volunteers to further the screening. First, classify the adhesive prototypes from the highest to the lowest tack, peel and MVTR. Next, the appropriate carrier will need to be selected using flexibility, durability, weldability and breathability requirements.
During this stage of the development process, it might be time to determine which of the selected adhesives will be more prone to generate skin damage. The development of skin irritation is not an uncommon feature of long-term wearable adhesives and is usually related to skin stripping due to application and re-application of adhesive products.
Dykes published studies to evaluate the irritancy of the adhesive border of wound care products in volunteer subjects.3 The method includes measuring the transepidermal water loss (TEWL) and skin hydration before and after multiple re-applications. Both parameters can easily be measured using a Tewameter and a Corneometer. An increase in TEWL and skin hydration correlates to superficial skin damage. In addition, there is a known correlation between superficial skin damage and pain at removal; using this method can ease the selection of a less painful, less damaging adhesive.
The inner volar forearm is a practical site to use for conducting this test. It is easily accessible and has generally thinner and more fragile skin compared to other body areas, therefore representing a worst-case scenario. The re-application should be at least 4-6 hour intervals to allow the adhesion level to build up before peeling.
These steps are valuable when the device is to be re-applied multiple times in the same location. For devices that are only applied once, the evaluation of the skin stripping at removal should be done over the course of the user study.
Volunteer Study
Based on the classification of the adhesive, a study on healthy volunteers should be conducted with the wearable device itself (or a surrogate that has similar weight, shape and thickness) at the application. While it might be tempting to conduct a study without the device, its stiffness and weight, as well as management of the moisture underneath the device, are all key factors that will impact the adhesive performance and must be evaluated accurately for effective results.
The study on healthy volunteers (user study) should be set up in such a way as to have sufficient application sites per prototype; a minimum of 15 sites is recommended to be able to see a difference between the adhesive options. To increase the power of the study, extend the study further than the expected wear time of the device so that more precise data can be obtained. It is also fundamental to randomize the location of the samples, as well as do a close follow-up of the study to determine:
• Is there any discomfort or pain reported by the panelist?
• How did the sample lift?
• Is there any residue around the carrier?
• Is there skin stripping at removal?
In the event that the user study yields unexpected results or results that don’t match the acceptance criteria, reverting to rheology data and MVTR generated earlier in the development cycle should support the selection of an improved alternative. Finally, once the results of the study are satisfactory, it will be time to begin the clinical study necessary for claim support and commercial launch.
Look Long Term
While proper screening and testing to choose the optimal wearable adhesive may seem work intensive, it can actually save time and money by reducing risk in the long run. Designers often tend to move forward with an existing adhesive carrier substrate without careful evidence, which might lead to failure at the clinical stage entirely because of improper device adhesion. ASI
For more information, visit www.scapahealthcare.com.
References
1. K.W. Ho, K. Dodou. 2007. “Rheological Studies on Pressure-Sensitive Silicone Adhesives and Drug-in-Adhesive Layers as a Means to Characterize Adhesive Performance.” Int. J. Pharm. 333, 24-33.
2. M. Haber. 2013. “Human Skin Surface Model Material Substrate for In-Vitro Adhesion Assays.” European Cells and Materials, Vol. 26. Suppl. 6, (p. 11).
3. P.J. Dykes. 2007. “The Effect of Adhesive Dressing Edges on Cutaneous Irritancy and Skin Barrier Function.” J. Wound Care. Vol. 13 N03.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







