Waterborne Acrylic PSA Technology with High-Temperature Performance
A new waterborne technology offers excellent adhesion properties and high temperature performance.
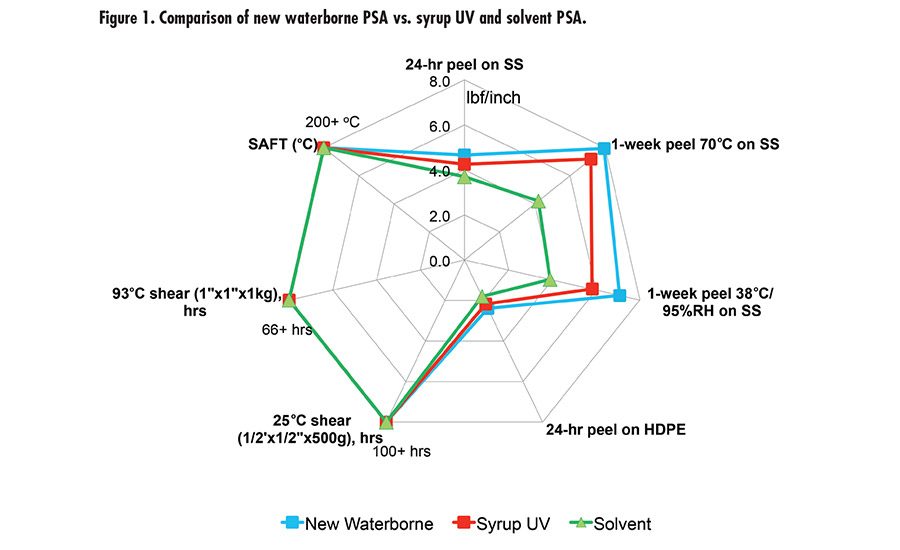
Fig. 1. Compairison of new waterborne PSA vs. syrup UV and Solvent PSA.

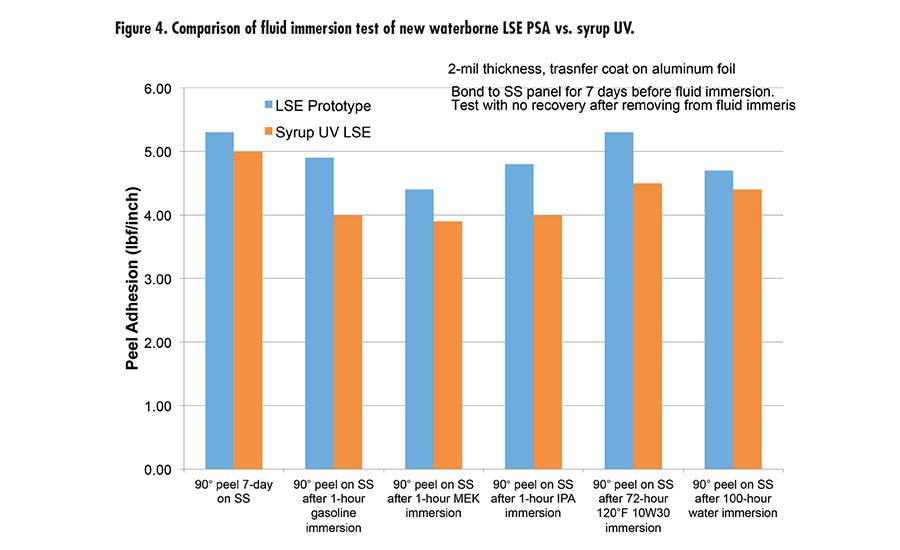
Fig. 2. Compairison of fluid immersion test of new waterborne PSA vs. syrup UV.








Pressure-sensitive adhesives (PSAs) are ubiquitous in many applications, such as labels, packaging tape, medical tapes, automotive tapes, and graphics. Two major chemistries are used in PSAs: acrylics and rubber in the forms of solvent-based, waterborne, or hot melt.
Waterborne acrylic PSA offers many advantages: good ultraviolet (UV)/thermal stability, low cost, low hazards, environmental friendliness, and high solids. Significant performance gaps between waterborne and solvent PSAs traditionally limit the use of waterborne technology in some more demanding applications. Industry has been pushing the boundary of the waterborne technology to close these gaps.
A new waterborne technology with excellent adhesion properties and high-temperature performance has been developed. This new technology has applications in the specialty tape market with emulsion technology; it matches and, at times, surpasses the well-established solvent acrylic and UV syrup technologies that dominate that space today.
Preparing the Material
Crosslinking between polymer chains is critical for PSA performance. There are some fundamental differences in the crosslinking between solvent-based and waterborne systems. Solventborne PSA is a homogeneous system with polymer chains dissolved in solvents. After the solvents evaporate, the crosslinking between polymer chains is uniformly distributed.
Waterborne PSA is a heterogeneous system with polymer particles dispersed in water, which makes the crosslinking a bit complicated. After the water evaporates, the polymer particles start coalescing for the film formation. The crosslinking can be intra-particle, inter-particle or both, depending on the crosslinking system. There can be some weak regions due to the inhomogeneity of crosslinking network.
Different types of crosslinkers and functional monomers can have different effects on peel adhesion and high-temperature performance. The high temperature resistance is measured by the shear adhesion failure temperature, or SAFT (PSTC-17 test method).
Semi-batch emulsion polymerization was used in preparing the waterborne acrylic PSA for this study. The polymer contains 2-ethylhexyl acrylate, butyl acrylate, methyl methacrylate, styrene, and acrylic acid with calculated glass-transition temperature (Tg) of -40°C. The samples were tested by coating onto 2-mil PET film, air dried at room temperature for 30 min and then further dried at 110°C in an oven for 3 min with a dried adhesive coat weight of 50 g/m2.
The adhesive films were cut into 1-in. strips for 180° peel adhesion testing on stainless steel (SS) panels with 15 min bond time, PL15 (SS), 24 hrs bond time, PL24 (SS), and SAFT test.
Effect of Crosslinkers
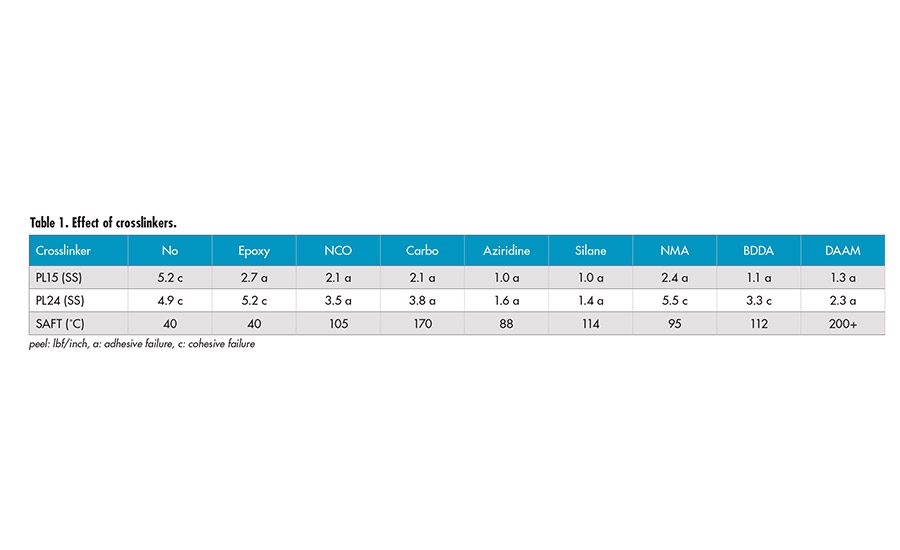
Several crosslinkers, including post crosslinkers and crosslinking monomers, were investigated. Multi-functional epoxy crosslinker appears to have little effect on the SAFT and adhesion, due to the requirement of high temperature for the crosslinking between epoxy group and carboxyl group of acrylic acid. Water-dispersible aliphatic polyisocyanate (NCO) can react with the carboxyl group to form amide and release CO2. The peel adhesion decreases and SAFT increases to around 100°C. The majority of the crosslinking may be inter-particle.
Polycarbodiimide (carbo) is a popular crosslinker used in water-based systems. It gives decrease in peel adhesion and significant increase in SAFT at 170°C. The crosslinking may be a combination of both inter- and intra-particle due to its hydrophobic nature. The tri-functional aziridine corsslinker can react with carboxyl functionality to form crosslinks at room temperature. There is significant reduction in peel adhesion and increase in SAFT at around 100°C. It seems that the crosslinking may be mostly inter-particle.
The trimethoxy silane group (silane) provides a possible mechanism for crosslinking during the film formation. There is significant decrease in peel adhesion and increase in SAFT to 114°C. Due to the hydrophilic nature after the hydrolysis of silane into silanol, the crosslinking is likely inter-particle.
NMA (N-methylol acrylamide) provides a self-crosslinkable system with pendant hydroxymethyl group. There seems to be very little decrease in peel adhesion and slight elevation in SAFT. We aged the film of the sample containing NMA at 50°C for 1 week and re-tested the sample. There is a very significant increase in SAFT to 200°C. It appears the crosslinking of hydroxymethyl group occurs at elevated temperature and has a strong effect on SAFT.
BDDA (1,4-butanediol diacrylate) is a di-functional acrylate monomer, which forms mainly intra-particle crosslinking. There is increase in SAFT to 112°C and peel adhesion is reduced. Interestingly, the 24-hr peel shows cohesive failure mode with BDDA crosslinker. The crosslinking inside the particles may have caused the poor coalescence. This may result in low integrity of the films and lead to the cohesive failure.
DAAM (diacetone acrylamide) is a room-temperature crosslinkable system with addition of ADH (adipic dihydrazide). The DAAM monomer was copolymerized into the polymer backbones. The carbonyl group of DAAM will react with dihydrazide to form crosslinking at room temperature after drying. There is a decrease in peel adhesion and strong increases in the SAFT. It appears to be an effective crosslinking monomer providing both intra- and inter-particle crosslinking due to its nature and slower reaction, allowing the crosslinking to continue after particle coalescence.
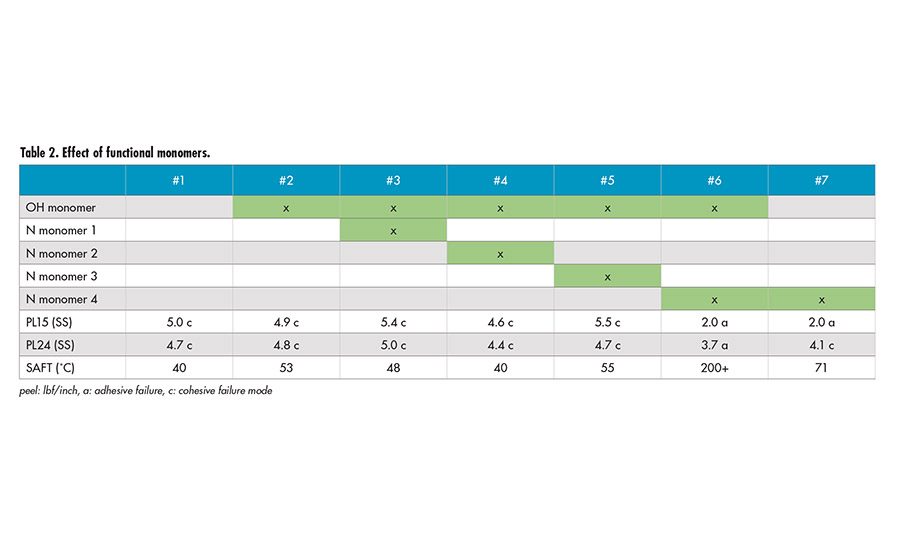
Effect of Functional Monomers
The effect of hydroxyl (OH) functional monomer and several different types of nitrogen-containing (N) functional monomers were also explored to investigate the effect of different functionalities on SAFT. Table 2 shows minimum effect on SAFT with different functional monomers, except Sample 6. It appears the combination of hydroxyl monomer and nitrogen-containing Monomer 4 provides a very strong effect on SAFT. The presence of only nitrogen-containing Monomer 4 (Sample 7) and only hydroxyl monomer (Sample 2) does not lead to the drastic increase in SAFT.
Dynamic mechanical analysis (DMA) was used to characterize Samples 1 and 6. It was shown that there is an increase of storage modulus (G’) at elevated temperature for Sample 6. This is an indication of crosslinking reaction, which has led to the increase in SAFT. The actual mechanism of crosslinking is being further investigated.
Prototypes
Different crosslinking mechanisms can lead to different effects on high temperature resistance (SAFT). Fundamentally, there will be more to investigate in terms of structure/property to fully understand how different crosslinking mechanisms affect the SAFT. With this research work, we developed a new prototype waterborne acrylic PSA that has superior adhesion properties and high SAFT. The new waterborne PSA was benchmarked with a leading market tape product using monomer syrup UV acrylic technology and a solvent PSA, as shown in Figure 1. The new waterborne technology has comparable adhesion performance and SAFT vs. the syrup UV and solvent PSA.
The new waterborne PSA was also tested for immersion in different fluids, including water, gasoline, methyl ethyl ketone (MEK), isopropyl alcohol (IPA), and 10W30 engine oil (see Figure 2). This was done with 2-mil aluminum foil backing and tested with 90° peel. It exhibits great performance and peel retention after immersion.
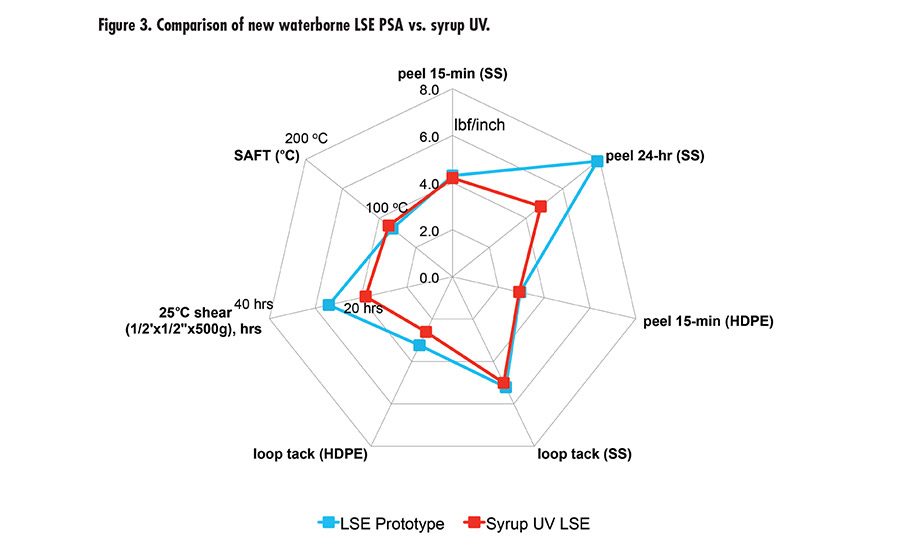
Due to the high peel and high shear of this new waterborne technology offers, we took it to the next level with tackification and developed a new LSE prototype with excellent adhesion to low-energy surface, tack, and high shear. The LSE prototype was benchmarked with a market-leading LSE tape product using UV syrup technology. Figure 3 (p. 27) shows the performance of the new LSE prototype and UV syrup LSE with very comparable peel, tack, and shear. It was also tested for peel retention after immersion in different fluids (see Figure 4). The results are excellent compared to UV technology.
Conclusion
New waterborne acrylic technology was developed with excellent adhesion performance and high temperature resistance. The LSE prototype derived from the technology also exhibits great balance of tack, peel and shear. Both products have great peel retention after immersion in different fluids. This opens the door for the waterborne acrylic PSA to be used in high-end specialty tape applications. These products have higher solids levels than most solvent acrylics, which improves coating efficiency. They also have performance that matches or exceeds the best-in-class offerings in specialty tapes today. ASI
For more information, visit www.synthomer.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!














