Designing Rebondable Structural Adhesives
Aromatic thermosetting polyesters have been shown to be fully compatible with the reversible bonding adhesion concept.

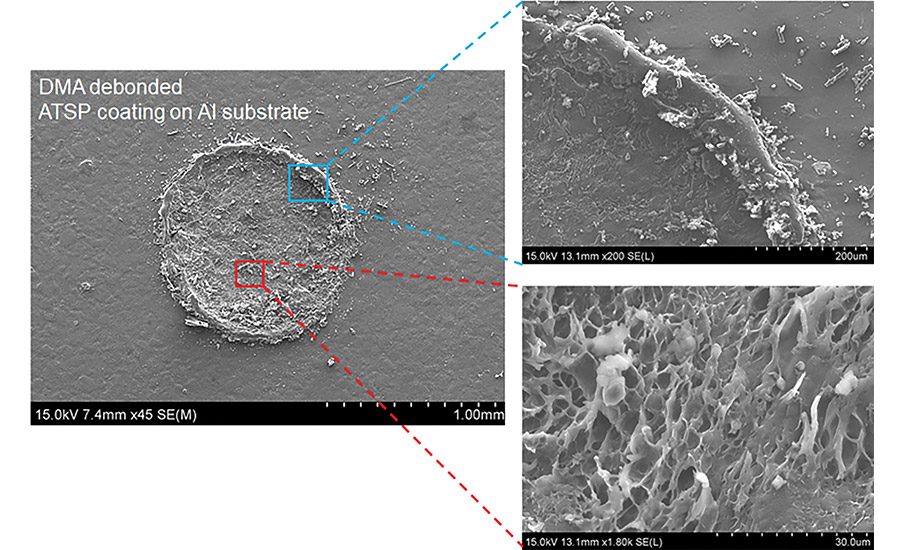
Figure 1. Cohesive failure.




Reversible adhesion inspired by the gecko has attracted attention in applications such as biomedical masks, climbing robots, transfer printing, and pressure-sensitive adhesives. The approaches used to accomplish reversible adhesion are typically classed into two categories: patterned and non-patterned. The first category uses a micro-patterned surface that mimics well-known biostructures, while the latter category does not.
The adhesion force between the surfaces is generated from contributions by Van der Waals, capillary, mechanical interlocking, covalent bonding, and hydrogen bonding. Greater compliance/conformability can be achieved by the patterned surfaces, allowing these adhesives to exhibit better adhesion than their non-patterned counterparts. This approach has been extensively studied in academia but has not found widespread use in industry.
Seeking Reworkability
Reworkable adhesives include thermoplastics that melt at an elevated temperature and solidify on cooling. Rework is accomplished in the melt state and allows debond and rebond/repositioning of components. Thermoplastics, in general, do not make great structural adhesives due to this melting behavior and creep (a process that can be thought as very slow melting under stress).
Another category of adhesives that is extensively used in the world of structural adhesives is thermosetting polymers. Thermosetting adhesives form chemical crosslinks on curing and do not demonstrate a melting point at any temperature, rendering them not reworkable.
One approach to the design of rebondable thermoset adhesives is through optimizing the chemical structure. This design requires the crosslinked thermoset structure to possess bonds that are labile (e.g., the bond connectivity is exchangeable between segments). Academically, these kinds of bond exchange reactions have been shown to occur even within fully thermoset networks.
Such reworkable thermosets with labile bonds would be fully solid-state, involving no melting (in the traditional sense). Even at temperatures well above the glass transition temperature (Tg), the material would be in the rubbery state and still formally a solid. When two surfaces possessing exchangeable bonds are put in contact with each other and a pressure is applied between the layers with a temperature sufficient to activate the bond exchange (in all cases above the Tg), the counterpart surfaces are joined and a fully contiguous thermoset network can be formed.
If we assume that the adhesive layer on both components possesses a high density of the exchangeable bonds throughout, then there is no real requirement for the debonded surface to exactly match the original profile to remain reversible (as in the case of gecko adhesives). Potentially, any area of cohesive failure will still expose an area with exchangeable bonds present on the surface.
Incorporating Aromatic Thermosetting Polyesters
In principle, crosslinked networks with labile bonds can be made to rebond if the coated layer does not delaminate from its substrate. Reversible bonding is defined here to be the repeated bond/debond process that breaks and reforms the bonds between two surfaces. Therefore, the critical factor is the strength of the coating on the substrate—sufficient substrate preparation must be used such that the coating does not delaminate.
Aromatic thermosetting polyesters (ATSPs) fit this description, as they contain ester linkages that are labile when heated above the Tg.1 Previous studies have demonstrated the interchangeability of these bonds. Secondary ion mass spectroscopy (SIMS) studies on deuterated and non-deuterated fully crosslinked ATSP resins have shown that the inter-diffusion distance was less than 50 nm, with neutron reflectivity studies. These results serve as a demonstration of joining of fully cured articles mediated by bond exchange reactions.2
Recently, ATSPs have been shown to be fully compatible with this reversible bonding concept. ATSP is first deposited via electrostatic powder coating onto the target substrates, and the coating is cured. The components are then brought together under heat and pressure, and a fully contiguous network between the coatings is formed.
ATSP is a high-Tg, high-thermal-stability polymer, so the operative bonding conditions are typically at 340˚C; top-end structural performance reaches to 400˚C. A powder-coatable adhesive additionally offers automated large-area uniform application, as well as a non-tacky and easy-to-handle condition during fabrication. Ready-to-bond parts can be shipped for bonding at an arbitrary later date. This means that the coated components, as treated as an adhesive, have an indefinite open time. In serving as an on-demand adhesive, it rapidly (< 1 min in high heat flux cases) attains full bond strength and allows inspection immediately upon cooling. The reversible bonding can help in reconfiguration of structures at a later date and with the recycling of parts, and it offers users of structural adhesives manufacturing flexibility and potential for rework.
Exploring In-Space Assembly
In a recent NASA-funded Phase I SBIR examining applicability for in-space assembly, a series of bonding cycles was imposed on aluminum coupons in a self-aligning geometry coated with an ATSP resin. For 51 cycles, the resin was bonded and broken. Following these cycles, a cohesive failure percentage of over 95% was observed, along with no decline in strength for the entire process history (see Figure 1). Throughout the set of cycles, the ATSP adhesive demonstrated mixed tensile/shear strengths of > 15 MPa (see Figure 1).
This bonding concept could potentially offer a solution for in-space assembly. At a glance, state-of-the-art space frame construction technologies3 have generally relied on the use of metal-based unit elements4,5 that are either permanently joined or connected via joint mechanisms that are labor intensive and difficult to automate. Although such designs are readily used on low Earth orbit space missions, their large weights and infeasibility of reassembly under space conditions becloud their implementation on future space missions.
To address these problems, recent studies have employed fiber composite unit elements attached via mechanical interlocks to build lightweight, high-strength/stiffness cellular structures.6 Even though these designs address the previously mentioned issues and present reversible joints, the length scales are currently far below those of targeted technological applications, and joining requires complex mechanical interlocks that may inhibit a fully autonomous assembly. Present automation concepts for assembly of cellular structures7 involve unit members that have a relatively low packing factor and specific mechanical properties, and therefore would needlessly occupy launch volume and mass. In addition, reconfigurable concepts that switch from one unit cell type to another have not been explored.
Regarding a bonding approach for primary structures, however, a DARPA/Lockheed Martin X-55 Advanced Composite Cargo Aircraft demonstration paved the way by illustrating the feasibility of building an adhesively joined unitized composite skin for a fuselage structure.8 Utilizing such an innovative approach, substantial weight savings on an aircraft frame were enabled by simply eliminating rivet and fastener use (more than 85%), along with significantly reduced material cost, time, and labor for fabrication and assembly.9
Several additional criteria are involved for a practical reversible adhesive scheme relevant to missions in space. The first is the need for a fully reversible and all-solid-state process, as liquids generally have an unacceptably high vapor pressure in vacuum. This eliminates approaches that rely on uncured polymer9 or a meltable interstitial phase.10
The second consideration is that the joint members of the structure and the reversible adhesive do not experience a glass or melt transition within the range of temperatures experienced by the structure during day/night cycles (-160 to 120°C) in conditions without thermal controls, which would induce undesirable adhesive reversion due to an uncontrolled change in phase and negate mechanical properties of the bonded interface.11,12 This presents challenges for shape memory polymers13 and some “gecko” adhesive14,15 schemes that generally rely on polymers that have glass transitions below 120°C. In addition, the reversible adhesive joint must be scalable and able to be implemented into complex geometries. This eliminates gecko adhesive schemes available in the literature16 due to their reliance on patterned fibrillar surfaces.
With this in mind, a solid-state adhesive approach based on covalent bond exchange within a high-temperature polymer presents itself as an attractive solution. A crosslinkable all-aromatic polyester such as that found in the aromatic thermosetting copolyester resin systems fulfills these criteria as a means of employing an adhesive in low Earth orbits and beyond.
For more information, contact the lead author at (217) 778-4400 or jacob.meyer@atspinnovations.com, or visit www.atspinnovations.com.
Author’s note: This research work was supported by NASA-SBIR program under grant contract NNX17CL41P. The SEM analysis was carried out at the Center for Microanalysis of Materials at the University of Illinois Urbana-Champaign, which is partially supported by the U.S. Department of Energy under grant DEFG02-96-ER45439.
References
- D. Frich, K. Goranov, L. Schneggenburger, and James Economy, “Novel High-Temperature Aromatic Copolyester Thermosets: Synthesis, Characterization, and Physical Properties,” Macromolecules, 1996, 29, 7734-7739.
- D. Frich, A. Hall, J. Economy, “Nature of Adhesive Bonding via Interchain Transesterification Reactions (ITR),” Macromol. Chem. Phys., 1998, 199, 913-921.
- NASA Report, “Reference Guide to International Space Station,” 2010.
- S. Rawal, “Metal-Matrix Composites for Space Applications,” Journal of Materials, 2001, 53, 14-17.
- D.B. Miracle and B. Maruyama, “Metal Matrix Composites for Space Systems: Current Uses and Future Opportunities,” Proc. National Space and Missile Materials Symp., ed. M. Stropki, Dayton, Ohio, Anteon Corp., 2000.
- K. C. Cheung and N. Gershenfeld, “Reversibly Assembled Cellular Composite Materials,” Science, 2013, 341, 1219-1221.
- MIT Center for Bits and Atoms Digital Materials and Assemblers, https://gitlab.cba.mit.edu.
- D. Kaufman, “Advanced Composite Cargo Aircraft Makes First Flight,” June 4, 2009, www.amc.af.mil/News/Article-Display/Article/147235/advanced-composite-cargo-aircraft-makes-first-flight.
- G. Gardiner, “Building TRUST in Bonded Primary Structures,” Composites World, April 1, 2015, www.compositesworld.com/articles/building-trust-in-bonded-primary-structures.
- X. Luo, K. Lauber, and P. Mather, “A Thermally Responsive, Rigid, and Reversible Adhesive,” Polymer, 2010, 51, 1169–1175.
- T. Xie, J. Hulway, and X. Xiao, “Shape Memory Polymer and Adhesive Combination and Methods of Making and Using the Same,” U.S. patent 8685528 B2, 2009.
- T. Xie, “Recent Advances in Polymer Shape Memory,” Polymer, 2011, 52, 4985-5000.
- H. Mengand and G. Li, “A Review of Stimuli-Responsive Shape Memory Polymer Composites,” Polymer, 2013, 54, 2199-2221.
- M. Bartlett and A. Crosby, “High Capacity, Easy Release Adhesives from Renewable Materials,” Adv. Mater., 2014, 26, 3405-3409.
- D. King, M. Bartlett, C. Gilman, D. Irschick, and A. Crosby, “Creating Gecko-Like Adhesives for ‘Real World’ Surfaces,” Adv. Materials, 2014, 26, 4345–4351.
- M. Bartlett, A. Croll, D. King, B. Paret, D. Irschick, and A. Crosby, “Looking Beyond Fibrillar Features to Scale Gecko-Like Adhesion,” Adv. Materials, 2012, 24, 1078-1083.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








