Automating Medical Device Assembly
Medical device manufacturers can find success when they embed processes and systems in a holistic manufacturing concept that takes into account the entire value chain.

The medical technology industry is growing faster than others and is currently experiencing a real change: more and more innovative products are being developed to help patients and relieve the burden on the healthcare system in the long term. The biggest hurdle is that medical devices can only be brought to market once they have complied with all regulatory standards and then, at best, be manufactured fully automatically to avoid cost-intensive manual handling.
If automation is not (yet) a possibility, it must at least be ensured that workplaces and interfaces, where upstream and downstream processes take place, can be optimally integrated, considering all quantitative and qualitative aspects. However, the leverage is far greater when companies embed processes and systems in a holistic manufacturing concept that takes into account the entire value chain, from the delivery of raw materials and parts to the assembly and delivery of the finished medical devices.
Adhesive Joints for Medical Devices
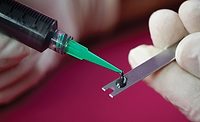
Special attention is paid to the assembly of medical devices, not only due to the high regulatory requirements but also because these processes often take place under clean room conditions. In the assembly of medical devices, adhesive bonding technology has gained in relevance as an innovative joining method. The electronics and automotive industries were the pioneers, as they recognized the advantages of bonding. Medical technology has now followed suit. The potential of adhesive bonding in medical technology has been recognized and will gradually replace classic laser welding.
Manufacturers of medical devices benefit from bonding in many ways:
- The thermal load on the joining materials is low or nonexistent. The materials of choice are UV adhesives or cyanoacrylates. Thermally curing adhesives are only used in special cases.
- The surface and microstructure of the components to be joined remain unchanged during bonding.
- An adhesive bond can also have sealing properties. If necessary, assembly processes can be streamlined and costs reduced.
- Almost the entire spectrum of component sizes can be bonded.
- An adhesive joint with UV adhesives allows repositioning of components during assembly. Using “active alignment,” readjustments can be made; only UV light hardens the adhesive and finishes the joining process.
Developments in catheter manufacturing show the potential of adhesive technology as a component of automated production. There is hardly a medical field in which catheters are not used, including in intensive care medicine for ventilation, in urology as bladder catheters, and in cardiology as heart catheters.
In simple terms, the production of a catheter always begins with the extrusion of the tubes, which in the next step have to be glued to the so-called connectors. This adhesive connection must also ensure that the catheter is leak-proof. Optimal production combines extrusion, cutting, and gluing via an automated process since the risk of error is highest in manual joining.
Volumetric Dispensing
One way to automate the joining process is to use purely volumetric dispensers, which enables continuous and pulsation-free dosing.* In addition, the process is gentle and low-shear with all materials regardless of the viscosity of the adhesives to be processed.
Particularly important for medical devices that are used on and in the body: smooth and sterile surfaces, the base of which are superimposed over the bonding and subsequent sterilization. The thread breakage of the adhesive, which is absolutely necessary for this, results from the technical implementation of the volumetric dosing unit: the conveying direction can be reversed. In this way, in addition to the exact thread breakage, material dripping is prevented.
Bonding of even the smallest, difficult medical devices is made possible due to the dispenser’s ability to convey and apply with an accuracy of ± 99%, even in the microliter range. The smallest amounts of adhesive are applied to the tubes without tearing and with pinpoint accuracy; the volume is demonstrably the same throughout the entire production cycle. The delivery rate can be changed if required for a specific component of the catheter (e.g., balloon, cuffs, or connectors). Even after adjusting the delivery rate, the dosage is accurate and tear-proof. In order to be able to cover all automated, semi-automated, and manual processes with one dosing technology, the dispenser has been technically designed for all areas of application.
In fully or partially automated processes, dispensers can be connected to the higher-level control system via a controller (foot switch). The operators have both hands free, which makes work more efficient while making the process more secure.
*Available under the names ViscoTec and preeflow.
Additional Medical Applications
Needle bonding is another application in which volumetric dispensers can provide fast and precise dosing of adhesives, auxiliary agents, or even active ingredients. Additional examples include the application of silicone on wound dressings and the lubrication of cannulas for smooth, low-resistance use of needles and intravenous vein catheters. Dispensers are also used to dose blood-separating gels into vessels used in blood collection, for example. Further applications can be the filling of the smallest amounts of “dermal fillers” or the filling of dental implants or dentures with two-component silicones.
Needle bonding in particular demonstrates the importance of the exact, tear-proof application of the smallest amounts of adhesive: here, so-called hollow needles (colloquially called cannulas) are bonded to the colored syringe head. In clinical use, the color of the syringe head indicates the outer diameter of a cannula, measured in gauge. A yellow cannula has an outer diameter of 0.3 mm, the specification for a dosing device that bonds such a hollow needle to the syringe head. It must be perfectly secure while also creating a leak-proof bond, as a leakage of liquids being administered to the body could lead to improper administration with potentially fatal outcomes.
The issue of tightness also plays a central role when, for example, optoelectronic components such as cameras, lights, or lenses are bonded into endoscopes. This type of medical device can only work 100% correctly if the bond has a perfect fit. The situation is also similar with hearing aids.
Most people think of smart watches when it comes to wearables, but one important market for wearable devices is medical and healthcare. Wearables already fulfill several diverse functions of medical devices, including monitoring vital functions like heart rate, oxygen level, body temperature, and respiratory rate.
State-of-the-art wearables provide great reliability and precision in a small package. Particularly in terms of comfort, flexibility, and connectivity, wearables are often superior to conventional medical devices. These technology advancements are possible through progress in sensor technology, power management, and transmitter technology.
The production method must also make progress to achieve these advancements, including with fluid management. In most wearable production processes, fluids such as adhesives, silicones, and thermally and electrically conductive materials must be applied fully automated with a high degree of repeatability. To be successful in all of these applications, the material must allow for sterilization after bonding and must also be safe for use on humans.
An alternative to bonding is the microencapsulation of components. Used to protect components in medical technology, encapsulation insulates and protects components from temperature fluctuations and ensures long-term stability during permanent or selective use in and on the human body.
Approvals and Qualifications
Whether bonding or microencapsulating, the combination of dosing technology and official approval (e.g., by the U.S. Food and Drug Administration, FDA) of the materials used forms the basis for reliably serving the medical device market of the future. Ultimately, when developing new adhesives and application technologies, the qualification of components and validation of the process are of great importance. This begins with fluid management and emptying the container in which an adhesive is delivered; it also includes degassing and the technical system for application.
With the knowledge of these basic conditions, medical device manufacturers can automate dosing, bonding, and micro-casting. This advancement will enable them to close a gap in many instances from partially automated to fully automated production processes while taking into account all national and global specifications by the relevant authorities.
For additional information, visit www.viscotec.com or www.preeflow.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




.jpg?height=200&t=1672772974&width=200)
