Pressure-Sensitive Adhesives (PSAs)
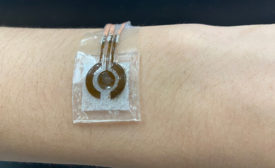
Adhesive Enables Noninvasive Glucose Monitor
Researchers at Penn State have developed a wearable glucose monitoring device prototype consisting of an electronic sensor attached to a small alkaline solution chamber.
March 23, 2022
Top 5 News that Sticks
Readers Drawn to Acquisition of Ashland Performance Adhesives
Last week, news of a completed acquisition drew the most reader interest.
March 21, 2022
Top 5 News that Sticks
H.B. Fuller Retains Top Position
Readers last week continued to be interested in H.B. Fuller’s appointment of a new executive vice president and chief operating officer.
March 14, 2022
Rethinking Medical Device Adhesives: More than a Sticky Afterthought
Choosing the right adhesive early can actively contribute to building better medical devices.
March 14, 2022
Supply Chain Strategies
Do You Have Suppliers or Partners?
Partners are always prioritized over transactional suppliers during times of allocation and disruption.
March 11, 2022
Keep the info flowing with our eNewsletters!
Get the latest industry updates tailored your way.
JOIN TODAY!Copyright ©2024. All Rights Reserved BNP Media.
Design, CMS, Hosting & Web Development :: ePublishing