Adhering to Difficult Substrates with Silicone Adhesives
Substrates with reactive groups available for bonding make chemical bonding easier to achieve, while substrates with nothing to react to make adhesion difficult.






Substrates
Surface energy is the thermodynamic effect of how a liquid wets out on, or spreads across, a surface. Adhesion chemistry tells us that the better an adhesive wets out, the larger the surface area covered, thus allowing more reactive groups and making a stronger bond.
Low-surface-energy materials such as plastic do not allow adhesives to wet out, making them generally poor candidates for bonding. For example, PTFE, the basis for nonstick cookware, has a surface energy of 18 dynes/cm,1 and most plastics are under 50 dynes/cm. On the other hand, aluminum, a better substrate for adhesion, is closer to 825 dynes/cm.
Depending on the industry, the use of various substrates is more common. For instance, medical devices present a large growth area for polycarbonate.2 Because of polycarbonate's established molding operations, ease-of-mold and low weight, manufacturers are incorporating it in many new devices. Polycarbonate can be found in applications such as blood reservoirs for medical applications and automotive headlamp housings. Polycarbonate is often selected for its excellent biocompatibility track record, high impact strength and dimensional stability. For our experiment, we looked at Bayer's Makrolon 2658-1112, a general-purpose, FDA-quality-grade polycarbonate without an internal mold-release additive.
Polyetherimides (PEI) are showing up in a number of industries, including aviation and automotive. They can be found in temperature sensors, connectors, flex circuitry and circuit boards. GE Plastics' Ultem® has become synonymous with the chemical, which is well suited for extreme service conditions, as it retains excellent tensile and impact strength, ductility properties at 190°C, high glass-transition temperatures of 215°C, high-volume resistivity, and flame, radiation, and chemical resistance. However, its surface energy is 52 dynes/cm, making it difficult for adhesion. We looked at two GE Plastics materials, Ultem 1000 and Ultem 2100-1000, with a loading of 10% SiO2.
Polyamide, otherwise known as nylon, is another plastic with low surface energy, although it's slightly higher than polycarbonate. Polyamide is probably the most diverse thermoplastic, and can often be found in medical tubing, automotive wire harnesses, reservoirs, aviation control knobs, and even cable ties. Nylons provide long wear, are inexpensive and are chemical- and thermal-resistant. Nylon 6/6 and 6 are the most common types of polyamides, with the numbers referring to the number of methyl groups occurring on each side of the nitrogen atom. A Dow Vydyne ECO315, Q3211-(RED) was used for these experiments.
If you're a serious golfer, you most likely have titanium shafts on your clubs. In addition, because of its favorable strength-to-weight ratio, titanium has become a staple in the orthopedic, aerospace and aircraft industries. Titanium also offers excellent resistance to moisture and acid corrosion; because of the nature of its protective oxide film, it is 20 times more erosion- and cavitation-resistant than copper-nickel alloys.
Some applications require the use of an adhesive capable of bonding metals such as stainless steel, aluminum or titanium. Examples range from the bonding of metal housings and turbine blades in aircraft engines and components to sealing pacemakers. Titanium and stainless steel are often chosen for their strength, durability and proven biocompatibility, whereas aluminum can be easily processed through molding, casting or machining.
Polyurethane is one of the most common substrates in use. From catheters, anesthesia masks and medical tubing to boat-engine gaskets and roller skate wheels, its excellent abrasion and chemical resistance make it a popular choice.
Polyurethane can be modified for different durometers depending on application requirements, but it retains excellent impact strength at low temperatures. We used a Dow Pellethane 2103-55D, a common polyurethane, for these experiments.
Polymethylmethacrylate (PMMA), more commonly referred to as acrylic, can be found in various applications, including aircraft windshields, aviation instrumentation, lawnmower covers, and blood pumps and filters. Acrylic, or Plexiglass, is known for its excellent clarity and weatherability, and is often used in outdoor applications where an absence of yellowing or embrittlement is critical. We used CYRO Industries Cyrolite G20 100 for our testing.
Polysulphones are extremely chemically and mechanically stable and exhibit excellent thermal, electrical, and creep-resistant properties over a wide temperature range.
Weathering is poor but can be improved greatly with selected pigments. This material is common in housings and reservoirs, and aerospace and automotive applications, as well as components in business machines where high-temperature durability and electrical properties are important. Unfilled Polyethersulphone has a useful life of four to five years at 200°C, or approximately 20 years at 180°C. With reinforcing fibers like glass and carbon, high-demand applications - such as those that require continuous performance under stress above 200°C - can be accommodated.
DuPont High Performance Materials is a worldwide supplier of Kapton® polyimide film. Kapton has enjoyed more than 35 years of proven performance as the flexible material of choice in applications involving extreme high (400°C [752°F]) or low (-269°C [-452°F]), temperatures. Kapton is used in a variety of applications, such as substrates for flexible printed circuits, transformer and capacitor insulation, and bar code labels.3
Materials
Primers have become a necessary evil for adhering to difficult substrates. Though often used to aid in adhesion, primers do add another bit of "black magic" to the process. Silane primers are used to promote adhesion between two non-bonding surfaces. These primers are primarily used with silicone adhesives, but they can be used with other types of adhesives, such as epoxies. The primers usually consist of one or more reactive silane, a condensation catalyst and some type of solvent carrier. The reactive silane typically has two reactive groups: one that is compatible with the substrate and another with the adhesive. Some types of groups may be hydrophilic, like a silanol group, or hydrophobic, like a one-octenyl group. These different groups form a compatible interface between incompatible substrates, thus promoting adhesion. Reactive silanes are usually added as moisture-sensitive alkoxy silanes, which form the priming surface in the presence of water and a condensation catalyst.
The reactive species are typically found in concentrations of 5-20% in solvent. The main job of the solvent is to dilute the reactive species, silanes and condensation catalysts on the substrate's surface. Once the silanes and condensation catalysts are in position to form a thin polymeric film on the substrate's surface, they begin hydrolyzing with atmospheric moisture, and the condensation catalyst joins all the hydrolyzed groups into a primer film on the substrate. Some condensation catalysts, like organotitanates, are part of the primer film and also help promote adhesion.
Theoretically, the best primer film is a mono-molecular layer, with the compatible groups facing the substrate and the organic groups facing the organic silicone adhesive surface. In reality, these monolayers don't exist, but compatible bi- or tri-layers do. This illustrates the importance of thin primer films and the necessity of solvent carriers in primer formulation. Thick, overly primed surfaces tend to build chalky primer films that can become points of adhesive failure.
Application methods range from simply wiping the primer on a surface to covering the primer through a sprayer. The primer is applied in a thin, uniform film, allowing the solvent to evaporate and the reactive groups to hydrolyze and condense with no pooling or fisheyes. After the solvent evaporates, there must be minimal humidity in the air, typically 30-60%, as excess water slows or stalls condensation. The usual recommended minimum time for cure - from application to usage - is 30 minutes. It is possible to accelerate the cure with heat from 35°C to 80°C, but careful tests must be performed to assure the primed adhesion doesn't suffer during this process. The primed surface should then last a long time, provided it is protected from contamination or abrasion.
Primers are moisture sensitive, and poor bottle handling can affect performance. If the bottles are repeatedly opened, an effort must be made to prevent the introduction of water; likewise, humidity must be displaced with either dry air or inert gases, like nitrogen. Another method is to package primer in the smallest practical container, which minimizes the frequency at which a particular bottle is opened. Another consideration during application is the buildup of residue on applicators or spray heads. Over time, primer forms a chalky residue that can be transferred to parts by using old applicators. Spray heads can be partially or completely obstructed by this residue. For these reasons, some controls are required during application, such as changing applicators periodically or inspecting spray heads daily.
While some caution is required when working with silane primers, the end result may be greatly increased adhesion of previously non-bonding surfaces. Careful initial experiments and the use of systematic manufacturing procedures are needed to ensure successful primer application.
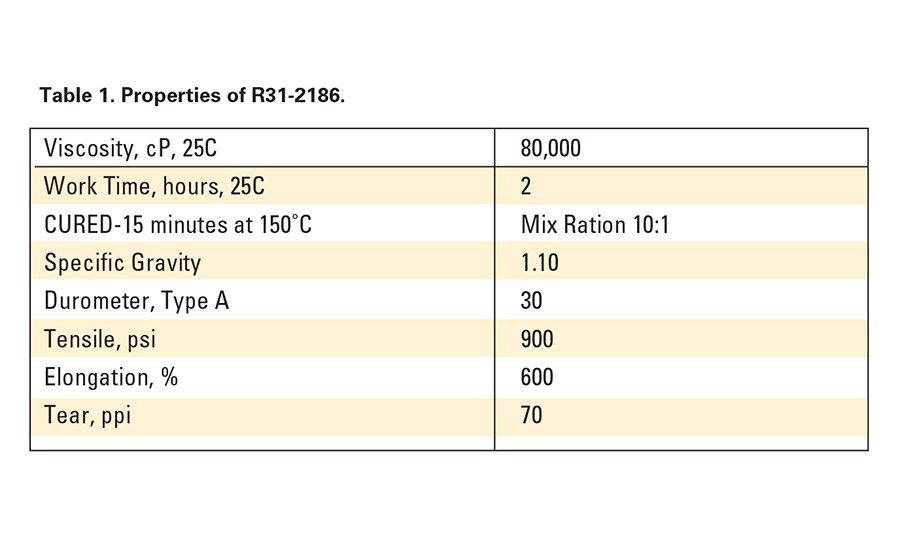
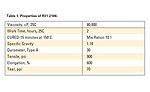
To promote adhesion to novel substrates, SP-270, a unique primer containing a proprietary blend of silanes, catalysts and solvents, was developed. R31-2186, a fast-cure addition-cure adhesive,4 was used in conjunction with this primer. Typical properties are shown in Table 1.
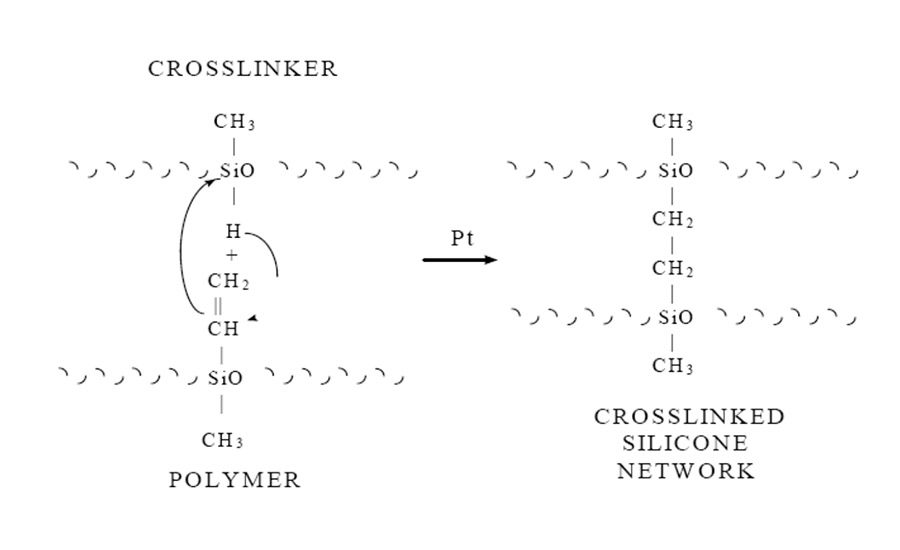
The cure mechanism for this addition-cure system involves the direct addition of a hydride functional crosslinker to the vinyl functional polymer, thus forming an ethylene bridge crosslink (see Figure 1). Because this mechanism involves no leaving group, unlike the one-parts, these systems can cure in closed environments.
Most platinum systems can fully cure at room temperature in 24 hours or can be fast-cured with heat. They can also be partially cured (tack-free) with heat and then packaged. Curing continues in the sealed package with no adverse effects, though it may be necessary to take special care to eliminate the presence of contaminants that could have a negative impact on the catalyst.4
Testing Parameters
Each substrate was cut into a lap-shear configuration of 1 x 4 inches. Six strips of each substrate were prepared to make three test panels, which were then cleaned with isopropanol to remove dirt, grease or particulates. SP-270 was added to a one-square-inch area on one end of each lap panel (as described above) and allowed to sit for at least 30 minutes. A bond thickness target of 5 ml (0.005 in) was used for applying R31-2186 to the primed area of the panels. The two panels were pressed together to form a sandwich, and careful consideration was given to not apply too much pressure to the bond surface. Sandwiched panels were placed in an air-circulating oven set at 70°C for a one-hour cure. ASTM D 1002 (Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading)—as is approved for use by the Department of Defense5—was the test method reference. The substrates were flame-treated and then primed with SP-270 silicone primer.
These difficult substrates are materials for which none of a variety of primers worked to improve adhesion. Also, none of a diverse assortment of adhesion techniques worked, including cleaning with different solvents, partially dissolving the substrate with solvents, boiling in water or abrading the polymer surface. Flame treatment of the substrate was performed via a propane flame to oxidize the surface of the substrate, which resulted in a high-energy surface conducive to bonding. Because flame generates excited species that attack the polymer surface, care was taken not to overheat the surface and cause damage. (A cooler flame is the better solution for preventing polymer damage.) Analysis indicated the presence of alcohol, acidic and carbonyl groups on the surface of the polymers. (It's important to note that flame treatment may also oxidize any hydrocarbon type contaminant.) The surface was then primed, and the material adhered.6
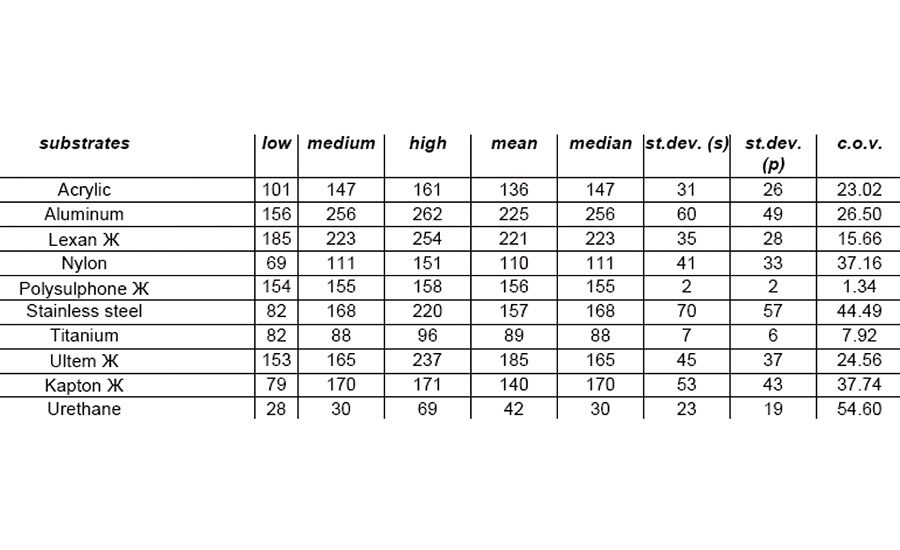
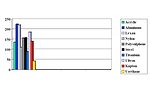
The equipment used to test for lap-shear value was an ISTRON Model 1011 with MTS data acquisition and 100-lb load cell installed. Results are shown in Table 2 and Figure 2.
Conclusion
The two predominant mechanisms of failure in adhesively bonded joints are adhesive or cohesive failure. Adhesive failure is the interfacial failure between the adhesive and the substrate, which indicates a weak-boundary layer—often from improper surface preparation or adhesive choice. Cohesive failure is the internal failure of the adhesive itself, indicating that the bonded materials' strength is greater than that of the adhesive's own physical properties; this is the preferred goal of any adhesive. Usually, the joint failure is neither completely cohesive nor adhesive, and measurement of a particular joint's success is based on the relative percentage of cohesive-to-adhesive failure.7
For this adhesive/primer system to have 100% cohesive failure with all the substrates tested was fantastic. Although lap-shear strength was not great with all substrates, such as titanium and urethane, these materials still worked for applications requiring the most basic adhesion. Given the breadth of substrates available, these adhesive/primer materials make good starting points and take the mystery out of some of that "black magic."
For more information on silicone adhesives, contact Nusil Technology, 1050 Cindy Lane, Carpinteria, CA 93013; phone (805) 684-8780; fax (805) 566-9905; or visit www.nusil.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!