Dibenzoate Plasticizers in Waterborne Acrylic PSAs
New blends of dibenzoates have recently been introduced for adhesives and other applications.
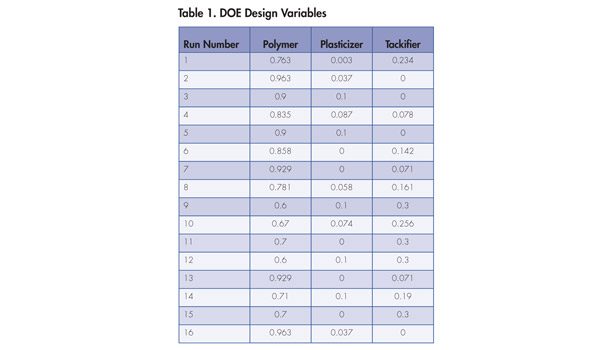
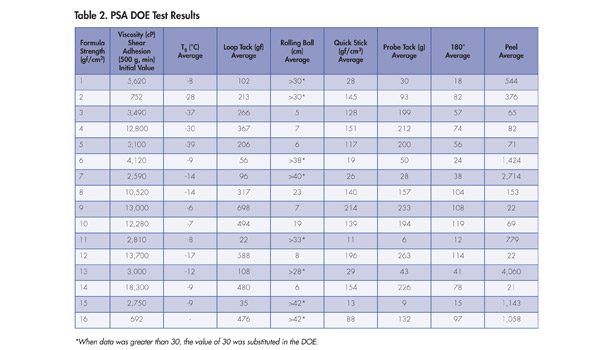
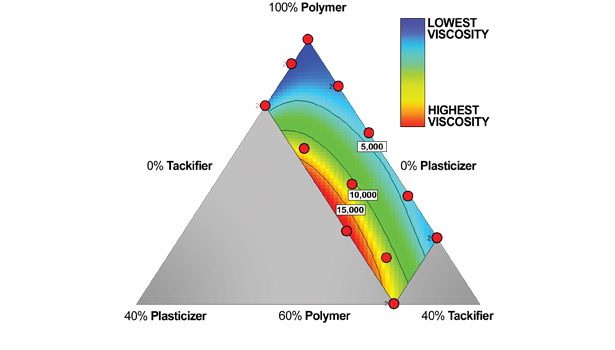
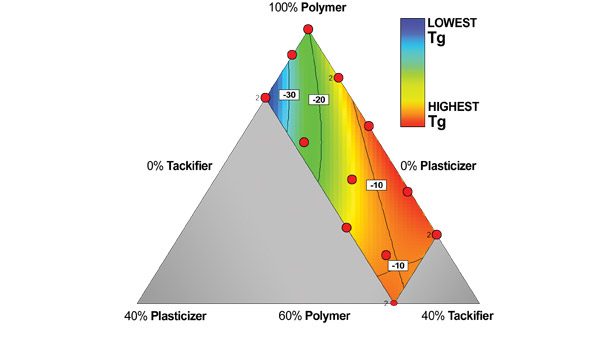
Figure 2. Glass Transitions (°C)


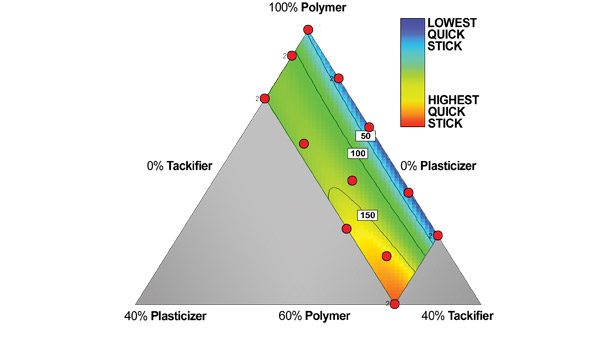
Figure 5. Quick Stick (gf/cm2)


Figure 7. 180° Peel (gf/cm2)









Plasticizers are normally used in polymeric systems to soften or to improve the processability of a composition. This is the basic definition of a plasticizer, with the caveat that the plasticizer needs to be at least partially compatible with the polymer being used. Because pressure-sensitive adhesives (PSAs) are a unique class of adhesives characterized by their ability to form bonds when light pressure is applied, additives such as plasticizers can play a key role in the formulation to enhance performance.
To measure a plasticizer’s effectiveness in a composition, the reduction of the glass-transition temperature (Tg) of the polymer can be used as an indicator; the degree of suppression of the glass transition is also an indication of the compatibility of the plasticizer with the polymer and the other ingredients. The workability or processability of a plasticized composition may be more important than the actual softening of the polymer for some applications, as is the case in PSAs.1
Plasticizer Performance
The use of plasticizers to improve the performance in PSAs is well known.2-5 The need for a plasticizer in a PSA is driven by the end-use characteristics of the adhesive, as well as the type of polymer on which the PSA is based. However, waterborne, acrylic-based PSAs, solution-borne PSAs and hot-melt PSAs have been known to contain plasticizers used for the purpose of enhancing performance. For example, plasticizers are used in waterborne, acrylic-based PSAs to:
• Improve the wetting of the substrate and speed of wetting
• Increase peel strength
• Reduce the effective softening point of a tackifying resin, so the combination will tackify more effectively
In addition to improving peel, the tack of the adhesive can be improved. However, plasticizers will reduce the holding power of the adhesive, which is an important consideration. Plasticizers will affect the performance of solution-borne acrylic adhesives in a similar manner. Plasticizers are used to control the service type of a PSA—to adjust the formulation’s overall Tg to meet the Dahlquist criteria for performance.6-9
Classically, di-n-butyl phthalate (DBP), diisobutyl phthalate (DIBP), di-2-ethylhexyl phthalate (DOP) or diisodecyl phthalate (DIDP) have been the plasticizers of choice in polar polymers used to make PSAs.3,4 Other polar plasticizer types, including dibenzoates, have been known to be used in these types of adhesives. In hot-melt PSAs, oils such as aliphatic, cycloaliphatic and aromatic types are employed as plasticizers.10,11 Performance is dictated by the compatibility of the components within the formulation (or the lack thereof).
In addition, the class of phthalate esters has environmental and health concerns on the regulatory front. If replacements are required, non-phthalates such as dibenzoates are more often the plasticizers of choice. However, even before phthalate replacement was an important consideration, dibenzoates were known to be polar specialty performance plasticizers.
A significant amount of literature is published on the use of oils in PSAs, but not very much is available on the use of polar type plasticizers in any PSA, with even less literature available on the use of dibenzoates in PSAs.10 With that in mind, polar PSA polymers are the most likely area of significant use for dibenzoates, and a paper was recently published on the use of dibenzoate blends in such PSAs.12
Historically, dibenzoates have been known since the 1920s. Most recently, new blends of dibenzoates—a diblend and a triblend—have been introduced for adhesives and other applications. The triblend is a blend of diethylene glycol dibenzoate, dipropylene glycol dibenzoate and propylene glycol dibenzoate. The purpose of this study is to demonstrate how dibenzoates function in waterborne acrylic PSAs and will focus on the use of this new dibenzoate triblend.
Experimental: Introduction
One PSA acrylic waterborne polymer was selected to be the basis of the adhesives of this study. It has a Tg of about -20°C and contains approximately 60% solids. One tackifier dispersion was selected that has a Tg of about 35°C and a softening point of 85°C. The plasticizer selected for the study was the triblend of dibenzoate plasticizers described previously, which will herein be abbreviated as 975P. A design of experiments (DOE) approach was selected to gain a better understanding of the trends on how the polymer, resin and plasticizer interact.13
Design Expert™ 8, made by Stat Ease®, was the software used to generate and analyze the DOE. A mixture design, Optimal D, quadratic, was selected. The design variables were:
• Polymer: 60-100% maximum
• Tackifier dispersion: 0-30% maximum
• Plasticizer: 0-10% maximum
The response variables were:
• Viscosity: initial, 1 day, 3 day
• pH
• Rolling ball tack
• Probe tack
• Quick stick
• 180° peel
• Loop tack
• Holding power
All raw materials were entered into the DOE as “wet” rather than dry ingredients. A paper that lists the actual design and all the results was published early in 2013 in the PSTC preprints, a selection of which is summarized in Tables 1 and 2, as well as the sidebar.12
Results and Discussion
The data from the DOE experiment is illustrated in Figures 1-6. The design points are displayed as the red dots within each figure; for the chart gradient, a red color indicates high values, and blue indicates lower values. The isobars indicate the values in the text box for the isobar. The corner DOE values give amounts totaling the parts to a sum of 1. In Figure 1, for example, the top corner shows a total of 1 for the polymer, indicating 100% polymer with no added plasticizer or tackifier. Moving straight down toward the base of the triangle, the minimum amount of polymer shown is 0.6, indicating 60% total polymer, with the balance made up of tackifier and plasticizer (in this case, 30% and 10%, respectively).
The first test data on the viscosity of the adhesives are illustrated in Figure 1. The contour indicates that, as plasticizer level increases, the viscosity of the adhesive is also increased, indicating compatibility.
The Tg of the formulations evaluated in the DOE are shown in Figure 2. As expected, the plasticizer content will depress the Tg of the adhesive, but—perhaps because of the resin in the system—it seems to be non-linear.
The enhancement of tack is another reason to consider adding plasticizer to a PSA. Loop, rolling ball and quick stick were methods by which tack was measured. Figure 3 illustrates the loop tack data, where the plasticizer addition had a positive effect on tack. Notice that the highest value of tack was at the maximum level of both plasticizer and tackifier (10% and 30%, respectively).
Rolling ball tack is illustrated in Figure 4. In the rolling ball tack method, a lower number is indicative of higher tack, as the value obtained is the distance a ball rolls down a length of tape; tackier tape will stop the ball at much shorter distances. There is a dramatic increase in rolling ball tack as plasticizer concentration goes up. The polymer had such poor tack by this measurement technique for low plasticizer or high tackifier levels that data points could not be attained, as the tape could not be made long enough to stop the ball from rolling. DOEs do not support discontinuities like this, so when the test exceeded the maximum length of tape tested, the data point value was set to that length (30 cm).
The quick stick data is illustrated in Figure 5. These data resemble the loop tack data. The highest value by this test is at the maximum of plasticizer and tackifier.
The next parameter is probe tack, which is yet another way to evaluate the tack of a PSA. Figure 6 shows this data. The addition of the plasticizer significantly increases the probe tack of the PSA evaluated.
The 180° peel data are illustrated in Figure 7. Plasticizer content does have an effect on the peel strength, where the highest peel values are found at high tackifier content and moderate plasticizer content.
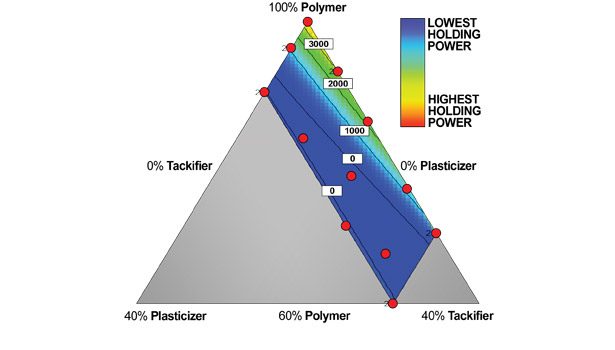
The holding power data is strongly influenced by the level of plasticizer or, to a lesser extent, tackifier used in the formulation. This is clearly shown in Figure 8. In this case, moderate holding power can only be achieved at lower levels of plasticizer and resin.
Conclusions
Plasticizer addition will definitely affect the performance of a waterborne PSA. In this evaluation, it was specifically observed that the dibenzoate plasticizer blend will:
• Reduce the Tg of the PSA. This change indicates that the plasticizer is compatible in the systems tested and is also a precursor to the other effects that were observed.
• Have an effect on the viscosity of the adhesive. In this case, the effect was an increase—this is also an indicator of the compatibility of a plasticizer in a waterborne adhesive, as well as a factor dependent on the solids of the adhesive under consideration.
• Increase tack. All of the measurements of tack that were employed showed that a plasticizer used in a PSA will affect the tack characteristics of the PSA. In this specific example, the effects were all positive. The type of polymer and resin used will certainly also dictate the magnitude of this effect or type of effect. A balance of the tackifier and plasticizer can achieve the performance required. The plasticizer will allow use of less tackifier if so desired and may also moderate the resin’s effects.
• Diminish the holding power of the adhesive. As is the case in all formulation design, what needs to be determined is the requirement for each parameter for each given application. These data may indicate that a higher Tg polymer with a higher molecular weight polymer best benefits from the use of a plasticizer if the tack is low.
• Affect 180° peel, which seemed to be the parameter least affected by plasticizer content. It was affected, but not as strongly as initially may be suspected.
It should also be pointed out that one dibenzoate blend (a blend of diethylene glycol, dipropylene glycol and propylene glycol dibenzoate) was used in this series of experiments. Dibenzoates are generally compatible with acrylic polymers, as well as the resins used to formulate waterborne PSAs; it would be expected that most of the dibenzoate family could be used in this application. The DOE data generated also indicate that it is possible to use this type of software as a tool to help tailor and potentially optimize formulation.
For additional information, visit www.emeraldmaterials.com.
References
1. Arendt, William D., “ASC Additives Short Course: Plasticizers,” November 7, 2007.
2. Satas, Donatas, Handbook of Pressure-Sensitive Adhesive Technology, Van Nostrand Reinhold Co., New York, NY, 1982, p. 325.
3. Satas, Donatas, Handbook of Pressure-Sensitive Adhesive Technology, Second Edition, Van Nostrand Reinhold Co., New York, NY, 1989, pp. 431, 470-472.
4. Satas, Donatas, Handbook of Pressure-Sensitive Adhesive Technology, Van Nostrand Reinhold Co., New York, NY, 1982, pp. 290-296.
5. Satas, Donatas, Handbook of Pressure-Sensitive Adhesive Technology, Second Edition, Van Nostrand Reinhold Co., New York, NY, 1989, pp. 329-331.
6. Horst, Roland H., “Rheological Analysis for Development and Quality Assurance of Pressure Sensitive Adhesives,” PSTC Tape Summit 35, 2012.
7. Petrie, Edward M., “Fundamentals of Pressure Sensitive Adhesives Part I – Types and Formulation,” Specialty Chemical presentation, September 5, 2012.
8. Chang, E.P., “Viscoelastic Windows of Pressure-Sensitive Adhesives,” The Journal of Adhesion, 34: 1, 189-200 (1991).
9. Satas, Don, “Correlation of Pressure Sensitive Adhesive Performance With Its Rheological Properties,” TAPPI Proceedings, 1989 Polymer Laminations and Coatings Conference, September 5-8, 1989, pp. 699-703.
10. Tsaur, Tom, “The Effect of Mineral Oil on Hot Melt Pressure Sensitive Adhesives,” PSTC TECH 32 Technical Seminar, pp. 59-70, 2009.
11. Arendt, William D., “Plasticizers in Hot Melt Adhesives,” Adhesive and Sealant Council Hot Melt Short Course, 2011.
12. Arendt, W.D., McBride, Emily L., Conner, Marianne M., “Use of Dibenzoate Plasticizers in Pressure-Sensitive Adhesives,” PSTC Tape Summit 36 Proceedings, May 15-17, 2013, New Orleans, Louisiana, pp 93-105.
13. Tkaczuk, Peter, “Statistical Experimental Design in the Development of Pressure Sensitive Adhesives,” TAPPI Proceedings, 1989 Polymer Laminations and Coatings Conference, September 5-8, 1989, pp. 705-720.
Acknowledgements
The authors would like to thank Ed Gotch, president and CEO, and Emerald Kalama Chemical, LLC for permission to publish and present this paper. We would also like to recognize Kate Williamson, Sarah Strother and Debbie Davidson for their help in preparing the data.
Disclaimer
The information contained herein is believed to be reliable, but is based upon laboratory work with small-scale equipment and does not necessarily indicate end-product performance. Because of variations in methods, conditions and equipment used commercially in processing these materials, Emerald makes no representations, warranties or guarantees, express or implied, as to the suitability of the products for particular applications, including those disclosed, or the results to be obtained. Full-scale testing and end-product performance are the responsibility of the user. Emerald Performance Materials shall not be liable for and the customer assumes all risk and liability for use and handling of any materials beyond Emerald’s direct control. Nothing contained herein is to be considered as permission, recommendation or as inducement to practice any patented invention without permission of the patent owner.
Experimental Details
- Preparation: The adhesives were prepared by mixing the plasticizer and resin dispersion into the polymer for 10 minutes at 750 RPMs using a Caframo® outfitted with a Jiffy® blade. Tapes were prepared on 1 or 2 mil polyester films with a 1 mil adhesive film thickness.
- Viscosity: The viscosity response of the emulsion to the plasticizers was determined at low shear by a
- 30-second reading at 20 RPMs on a Brookfield DVII, RV viscometer (22°C ± 2°C).
- Glass Transition: The Tg data was gathered on 10 mg samples. Samples were run by jumping from room temperature to -90°C and then ramped at 5°C per minute on a TA Instruments Q2000 DSC.
- pH: ASTM E70
- Rolling ball tack: ASTM D3121. Tapes were prepared of 5 mil (dry) films, 30 cm in length.
- Probe tack: ASTM D2979
- Quick stick: PSTC 5
- 180° peel: PSTC 101
- Loop tack: PSTC 16
- Holding power: PSTC 107-A
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!














